Metabolite Synthesis
Synthesis of Glucosides
Glucoside synthesis
Formation of glucoside metabolites of drugs is uncommon in humans, however they appear more frequently as plant metabolites of pesticides. Although glucosidation constitutes a relatively minor metabolic pathway in humans, the formation and urinary excretion of N-glucosides is important for some drugs such as barbiturates.
Hypha have a number of techniques for glucoside synthesis. This includes microbial biotransformation and a number of glucosyltransferases cloned from microbes in our collection. In addition, our late-stage chemical synthesis techniques are effective at making these conjugates.
We apply our one-stop metabolite shop techniques in parallel to determine the most efficient and cost-effective conversion to the glucoside of interest before proceeding to scale-up of each metabolite.
What We Do
Case Study - Synthesis of N-glucosides of an agrochemical
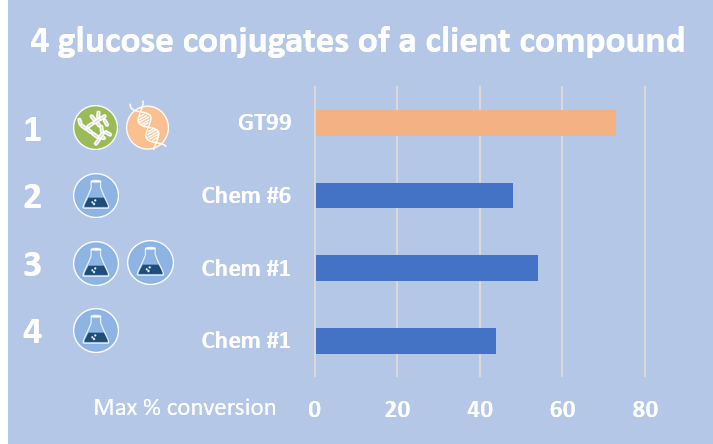
In this project, four glucoside metabolites of an agrochemical were synthesised.
Three glucosides were synthesised by late-stage chemical synthesis, with the other made by both microbial biotransformation and recombinant glucosyl transferases. All of these were produced in good yield.
For the glucoside conjugate made using microbial biotransformation and glucosyltransferase enzymes, the best conversion was achieved using a mutant created from the parent glucosyltransferase, more than doubling the conversion to over 70%.
Chemical synthesis produced 3 different glucosides using two chemical conditions that produced the conjugates at good conversions.
Find out about synthesis of other metabolite types
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
In recent years, FDA guidance has advised initiating human metabolite profiling earlier in drug development, emphasizing the importance of metabolite identification and quantification to evaluate a drug metabolite’s safety and pharmacological activity. Praliciguat (IW-1973) is a soluble guanylate cyclase (sGC) stimulator in Phase 2 clinical trials for diabetic nephropathy and heart failure with preserved ejection fraction (HFpEF). During studies on metabolism of praliciguat in preclinical species and in human hepatocytes, a prominent direct O-glucuronide metabolite was detected.
We’ve observed an increase in requests for synthesis of N-glucuronides over the last couple of years. We speculate that this may be due to the increasing use of N-heterocyclic chemistry in the design of new small molecule drugs, and pan company strategies to reduce CYP metabolism. The situation is further complicated by the high interspecies variability in formation of some N-glucuronides, especially aliphatic tertiary amines and aromatic N-heterocycles. UGT1A4 and UGT2B10 are key enzymes responsible for N-glucuronidation reactions in humans, rates of which can be much higher than in other animals. To compound this, synthesis of N-glucuronides is not always straightforward, and can be further muddied by metabolite stability issues, complicating interpretation of data.
The FDA’s 2020 MIST guidance states that phase 2 conjugates are generally pharmacologically inactive, however where a potentially toxic conjugate, such as an acyl glucuronide is formed, additional safety assessments may be needed. Idiosyncratic drug toxicity of carboxylic acid-containing drugs can be caused by the formation of reactive acyl glucuronides, which have the ability to directly acylate proteins and undergo intramolecular rearrangement producing reactive aldehydes leading to protein glycation.

Hypha Discovery did a fantastic job synthesizing N- and O- glucuronides of our clinical stage drug substance. The project updates were detailed, our questions were answered in a timely manner, and the overall timeline was maintained. Hypha was highly recommended to us and I would not hesitate to recommend them to a colleague.
Joshua Day Ph.D, Director of Chemistry
Crestone, Inc., Colorado, USA
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk


