AhR activators – friend or foe?
A very usual metabolite, a very unusual pharmacological target, and an unexpected serious adverse effect that combined to halt the development of laquinimod and roquinimex
By Andrew Parkinson, XPD Consulting
Laquinimod (Nervantra), a drug previously under development by Teva Pharmaceuticals for the treatment of multiple sclerosis, is converted by CYP-dependent N-de-ethylation to N-desethyl-laquinimod (DELAQ).
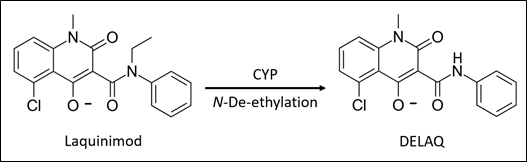
Figure 1: Metabolism of laquinimod to DELAQ
At the risk of tarnishing my reputation as a consultant, I have to admit that it took a long time to appreciate how unusual DELAQ is, and how different it is from laquinimod. Furthermore, circulating levels of DELAQ are a small fraction of laquinimod levels, so systemic exposure to DELAQ didn’t raise any flags. I couldn’t have been more wrong.
The significance of metabolism
Laquinimod is a non-linear anion (enol pKa[A] ≈ 5) with a LogP of ⁓2.6 and a topological polar surface area (TPSA) of 61 Å2. Due to internal hydrogen bonding, DELAQ is a linear, nonionized metabolite with a LogP of roughly 7. Circulating levels of DELAQ are so low compared with laquinimod because, as a nonionized metabolite with a LogP of ⁓7, DELAQ distributes extensively into tissues.
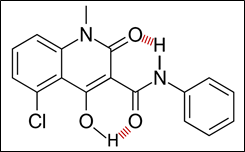
Figure 2: Internal hydrogen bonding of DELAQ
In contrast to laquinimod, DELAQ is a very potent activator of the aryl hydrocarbon receptor (AhR). In a mouse model of multiple sclerosis, laquinimod was pharmacologically active in normal mice but not in AhR knock-out mice, which established that AhR is the pharmacological target. Most AhR agonists are linear lipophilic compounds, so once you realize DELAQ has these properties due to internal hydrogen bonding, is it not so surprising that it activates AhR and confers pharmacological activity on laquinimod. In a 2021 publication, Rothhammer et al. disclosed that DELAQ itself cannot be administered orally because of its low solubility and short half-life, suggesting that laquinimod functions as a prodrug that is metabolized to DELAQ, its active metabolite, in the local microenvironment (see slides for citation).
Roquinimex (Linomide), a drug developed by Pfizer for the treatment of multiple sclerosis, is structurally related to laquinimod. It lacks a chlorine substituent and contains an N-methyl group instead of an N-ethyl group.
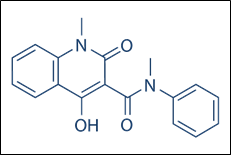
Figure 3: Roquinimex (linomide)
Clinical development of both laquinimod and roquinimex was halted due to serious cardiovascular events. This was unexpected. Studies in non-clinical species raised no obvious red flags of this clinical outcome.
Teva Pharmaceuticals licensed laquinimod from Active Biotech. Now that the former company has discontinued development of laquinimod for multiple sclerosis, the latter company is pursuing laquinimod as a treatment for non-infectious uveitis (https://www.activebiotech.com/en/projects/).
Whether activation of AhR is the mechanism by which laquinimod and roquinimex cause cardiovascular toxicity (including myocardial infarction) is not known. It may be an adverse effect related to exposure to parent drug or to metabolites other than the N-dealkylated metabolites.
Two take-home points
First, pay attention to the possibility that internal hydrogen bonding influences the physicochemical and pharmacological properties of parent drug and/or its metabolites.
Second, exercise caution developing a drug that activates AhR to a clinically relevant extent. We do not know why laquinimod and roquinimex cause cardiotoxicity, but it may be related to AhR activation.
A deeper dive
A more detailed description of this topic, together with references, can be found in these slides.
I have also included some slides that briefly discuss why general concerns surrounding AhR activation and induction of CYP1 enzymes are misplaced.
The fun slides are at the end.
About Andrew
Dr Andrew Parkinson is former Chief Executive Officer & Chief Scientific Officer of XenoTech, the contract research organization he founded in 1994 which has recently been acquired by BioIVT. Andrew has extensive experience based on testing thousands of drug candidates and consulting for over a hundred clients in America, Europe and Japan. He is currently a founding Consultant at XPD Consulting