Metabolite Synthesis
Synthesis of Stable-labelled and Radiolabelled Metabolites
Synthesis of Stable-labelled and Radiolabelled Metabolites
Hypha is experienced in labelled metabolite synthesis for challenging projects. We can produce both stable-labelled (2H or 13C) and radiolabelled (3H or 14C) metabolites of drugs and agrochemicals using techniques in our one-stop metabolite shop including microbial biotransformation, PolyCYPs enzymes and chemical synthesis.
For radiolabelled metabolites we partner with Selcia, where Hypha’s microbial and Selcia’s radiochemistry expertise permits production of radiolabelled metabolites which are difficult to synthesise chemically.
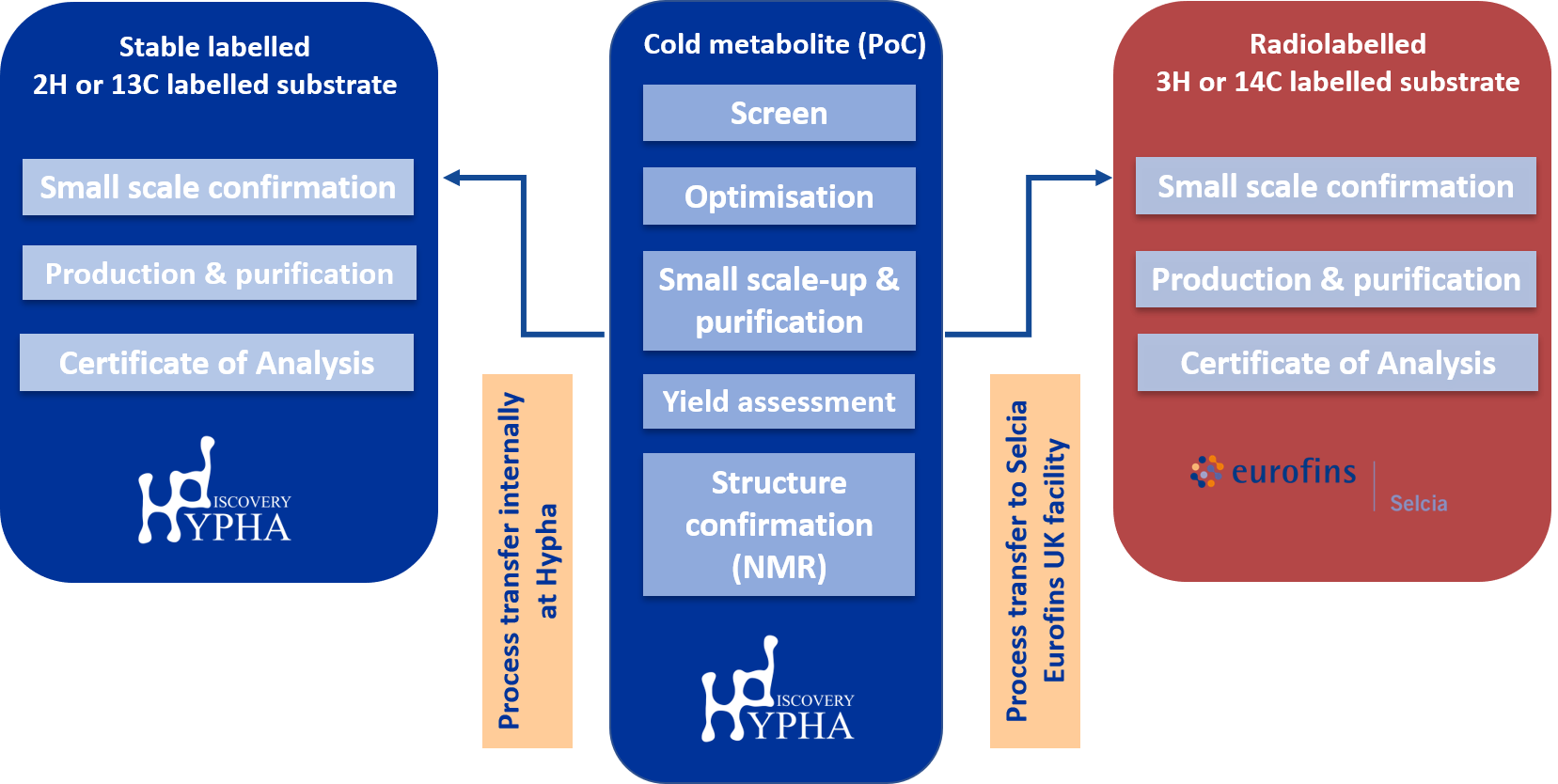
The first step in labelled metabolite synthesis involves identifying a process that will successfully produce the labelled metabolite.
For biotransformation methods, this involved screening for a microbial strain or PolyCYP enzyme which efficiently biotransforms the parent compound to the target metabolite, followed by a scale-up step to establish an optimised process and achieve acceptable yields of the product. Both the initial screening and scale-up steps are performed using unlabelled material. Structure confirmation by NMR spectroscopy is also performed at this stage.
Following successful completion of this process, incorporation of an externally-fed radiolabelled or stable-labelled parent drug to the selected microbial fermentation is then undertaken. For radiolabelled material, the optimised process is transferred by a Hypha scientist to Selcia’s radiolabelling labs for feeding and purification of the radiolabelled metabolite.
What We Do
Case Studies
Synthesis of deuterated metabolites
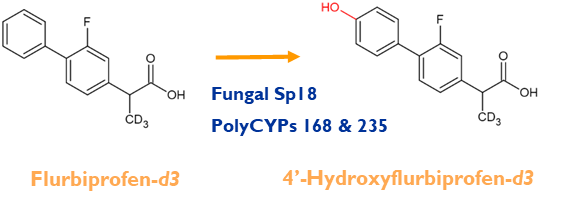
We can use late-stage chemical synthesis, microbes or PolyCYPs enzymes to make deuterated metabolites direct from the deuterated parent compound.
This case study features example projects in which we used biotransformation and chemical synthesis to prepare deuterated metabolites for clients.
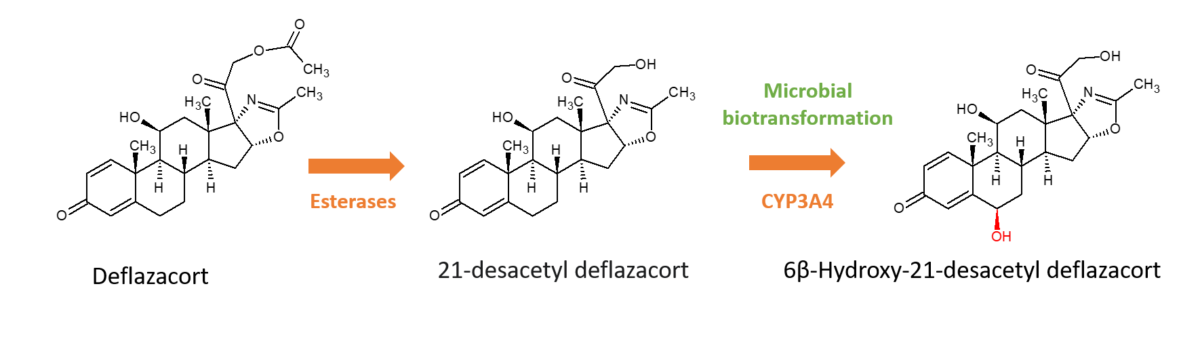
Deflazacort is a glucocorticoid which exists as an inactive pro-drug and then is rapidly converted by esterases to the active metabolite, 21‐desacetyl deflazacort, after oral administration. The active metabolite is then further metabolised by CYP3A4, primarily to 6β-hydroxy-21-desacetyl deflazacort (6β-OH-21-desDFZ). The pharmacokinetic drug interaction potential of 6β-Hydroxy-21-desacetyl deflazacort was not fully characterised and was subsequently required by the FDA post approval of the drug.
Chemical synthesis of 6β-OH-21-desDFZ is challenging since multiple steps as well as stereospecific reactions are required, which are time-consuming and costly. Microbial biotransformation was used instead to generate pure cold and radiolabelled quantities of the metabolite for CYP induction & inhibition studies and transporter interaction studies. This resulted in the generation of 62.1 μCi (equivalent to 448 μg) of the metabolite from 5 mCi of parent, with a radiochemical purity of 99.2% and HPLC-UV purity of 98.7%.
Availability of 6β-OH-21-desDFZ enabled studies which showed that the metabolite modestly inhibited CYP2C19, CYP3A4, MDR1, OATP1B1 and OATP1B3 activities. However, the metabolite was not expected to drive any clinically relevant drug–drug interactions due to the low plasma exposure at the therapeutic dose of deflazacort.
Find out about synthesis of different metabolite types

Hypha Discovery has been a valuable metabolite ID partner. Hypha have provided biotransformation, metabolite purification and structure elucidation answers to some of our most challenging metabolism and metabolite ID problems. We really appreciate the breadth of expertise available at Hypha Discovery and will definitely reach out for future work.
Director of DMPK
US Pharma Company
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
In this case study we look at how Hypha make deuterated metabolites, illustrated using case studies where biotransformation and late-stage chemical synthesis techniques were employed.
Hypha’s microbial biocatalysis process is effective at generating metabolites at up to gram scale. Through Hypha and Selcia’s partnership, [13C], [14C], [2H], [3H] and [15N]-labelled metabolites can be accessed to support regulatory, development or research projects in the pharma and crop protection industries. Hypha establishes optimized processes using unlabelled or stable labelled parent substrates, which can then be transferred to Selcia’s state-of-the-art radiochemistry labs for the production of radiolabelled metabolites.
Hypha’s PolyCYPs kits are in routine use by pharma and agchem companies for producing human and other mammalian metabolites. One application involves use of PolyCYPs for creating radiolabelled metabolites for direct comparison with the radio profiles from mass balance and distribution study samples, necessary for regulatory filing. PolyCYPs provides a clean route for scalable access to more of the CYP-derived metabolites observed in these matrices, for definitive MetID and any tox studies deemed necessary. This is especially useful where low concentrations or unstable metabolites in the mass balance sample make structural identification difficult.
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Supplier Code of Conduct Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved.