Late Stage Functionalisation
Deoxyfluorination
Deoxyfluorination
We can fluorinate drugs that contain hydroxyl groups, or hydroxylated metabolites of drugs where undesirable or excessive oxidation at certain positions has been observed during metabolic stability studies. Fluorination of metabolic soft spots is a strategy to reduce CYP-mediated clearance [1]. It may also confer other desirable properties due to fluorine’s unique ability to impact many important properties, including pKa, biological activity, lipophilicity, in addition to metabolism [2].
Hydroxylated intermediates can be made using late-stage chemical oxidation methods, electrochemistry, photochemistry, or by biotransformation using Hypha’s PolyCYPs enzymes. This can be done on multiple compounds in parallel.
PolyCYPs enzymes are used in our PolarExplorer service to provide a fast late-stage route to hydroxylate aliphatic or aromatic moieties at multiple sites in parallel, including metabolically susceptible positions. Both CYP-derived human metabolites and other oxidised derivatives can then be accessed simultaneously. This shortcuts the route to F-blocked metabolic soft spots by negating the need to confirm the structure of the hydroxylated metabolite – i.e. the deoxyfluorination reaction can be performed on the intermediate metabolite.
References
[1] Fluorine Analogs from Drug Metabolites. R. Scott Obach, Gregory S. Walker and Michael A. Brodney. Drug Metabolism and Disposition May 1, 2016, 44 (5) 634-646; DOI: https://doi.org/10.1124/dmd.116.069310
[2] Applications of Fluorine in Medicinal Chemistry. Eric P. Gillis, Kyle J. Eastman, Matthew D. Hill, David J. Donnelly, and Nicholas A. Meanwell. Journal of Medicinal Chemistry 2015 58 (21), 8315-8359. DOI: https://doi.org/10.1021/acs.jmedchem.5b00258
What We Do
Case Study
Generation of fluorinated derivatives of four drugs
Four drug compounds were subjected to 3 different late-stage defluorination conditions and fluorinated derivatives isolated and identified by NMR spectroscopy.
Two of these drugs (ezetimibe and rapamycin) already contain a hydroxyl group. The other two compounds are human metabolites of drugs mediated by cytochrome P450 metabolism (ruxolitinib via CYP3A4 and risperidone via CYP2D6). The hydroxylated metabolite of ruxolitinib was first generated using PolyCYPs enzymes, and paliperidone by chemical oxidation. In each case a fluorinated derivative could be made, purified and the structure confirmed.

The process of fluorination is an SN2 reaction so the stereochemistry is inverted. To access and test both enantiomers, a ketone derivative can be reduced enantioselectively and then converted into the enantiomerically pure fluorides.
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
Introducing oxygen into a drug candidate late in the optimisation process has several applications including exploration of SAR (structure-activity relationships) and the ability to access derivatives that may possess superior properties such as improved metabolic stability and LLE (ligand-lipophilicity efficiency). Biocatalysis can provide access to chemical space in a complementary manner to chemical synthesis and provide a “one-experiment” solution to accessing multiple derivatives in parallel. This poster illustrates the application of a new biocatalysis kit, PolyCYPs®, to enable parallel synthesis of hydroxylated derivatives of drugs.
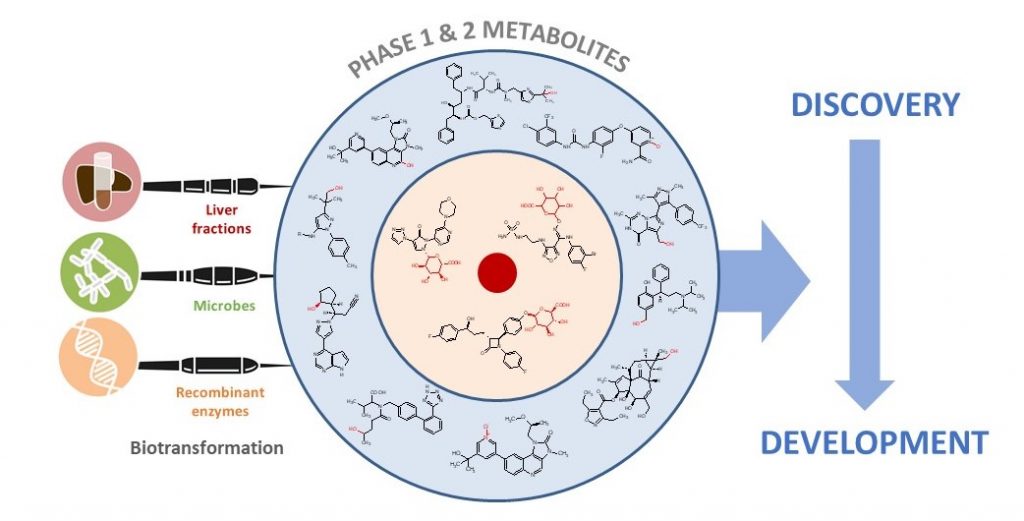
A cell-free kit of cytochrome P450 enzymes and ferredoxin/ferredoxin reductase redox partners, termed PolyCYPs®, is being developed for generating scalable quantities of oxidised metabolites. P450 cytochromes in the kit have been derived from some of Hypha’s most talented biotransforming bacteria and are capable of generating human and other mammalian metabolites of drug compounds.
In this paper, authors from Hypha and Incyte Corporation discuss the impact and application of biotransformation of drugs by mammalian systems, microorganisms, and recombinant enzymes, covering active and reactive metabolites, the impact of the gut microbiome on metabolism, and how insights gained from biotransformation studies can influence drug design.

The services conducted by Hypha allowed Alkermes to enhance our late stage functionalization efforts to rapidly expand our SAR understanding inside projects. In particular, the PolarExplorer work allowed facile generation of hydroxylated compounds possessing a complex molecular core. We look forward to engaging Hypha in the future because their team is professional, and their services complement our internal synthetic efforts for delivering new molecules.
Brian Aquila, Director of Medicinal Chemistry
Alkermes, MA, USA
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk