Metabolite Synthesis
Synthesis of Mixed Pathway and Multi-step Metabolites
Synthesis of mixed pathway and multi-step metabolites
Due to the broad xenobiotic capabilities of Hypha’s microbial screening panels, together with the addition of complementary late-stage synthetic expertise, we are able to make a range of metabolites derived from multiple pathways or sequential reactions using our one-stop metabolite shop approach.
Metabolites arising from sequential reactions require multi-stage synthesis; some are accessible via one-step whole cell microbial biotransformation, where both phase 1 and phase 2 reactions can be undertaken in the same fermentation as a consequence of the presence of both CYP/oxidative and UGT/glycosylating enzyme systems. This includes metabolites such as secondary glucuronides where, for instance, hydroxylation is first required followed by sequential glucuronidation.
Alternatively, where this is not possible, coupling microbial biotransformation with synthesis techniques permits the production of some difficult-to-synthesise sequential metabolites.
We also make metabolites of drugs or agrochemicals where mixed pathways are involved.
What We Do
Case Studies
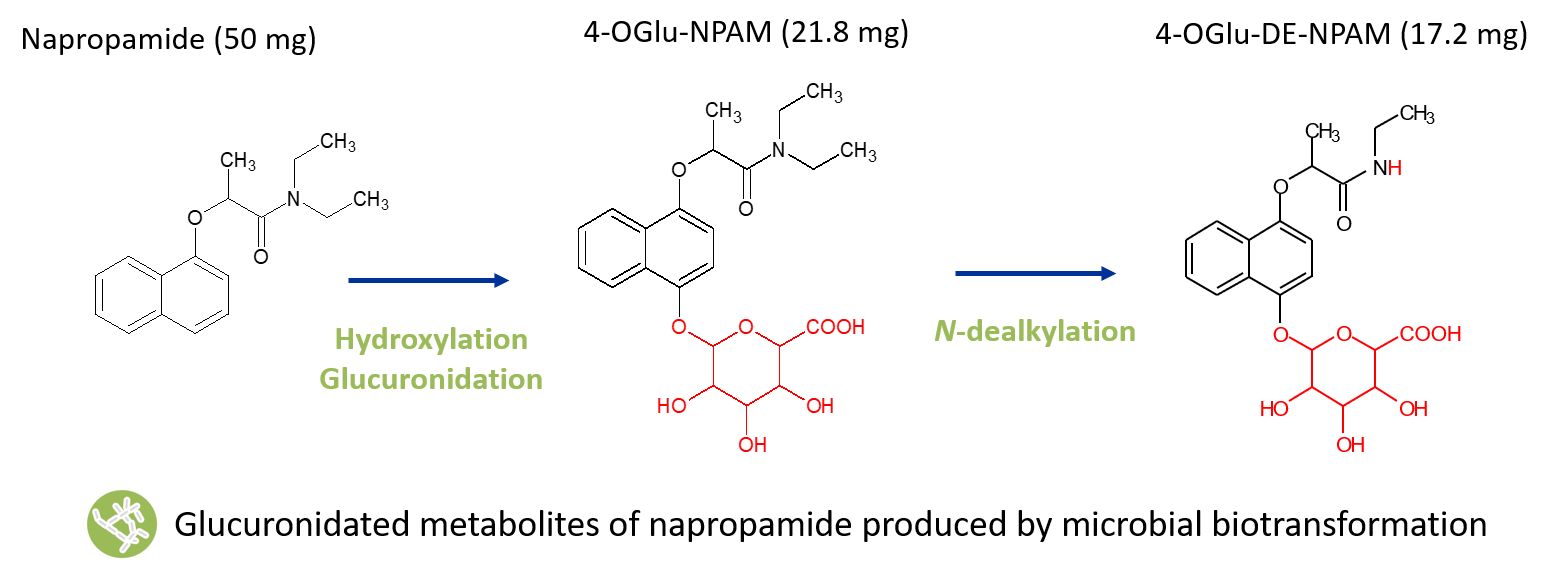
Hydroxylation followed by glucuronidation using microbial biotransformation
The herbicide napropramide is rapidly and extensively metabolised in animals, undergoing sequential aromatic hydroxylation and glucuronidation prior to excretion in urine and faeces (EFSA Journal 2010, 8(4): 1565). Napropamide was screened against a panel of Hypha’s biotransforming strains with several hydroxylated metabolites and glucuronides detected by LC-MS. A scale-up biotransformation enabled access to two of the major glucuronides relevant to animal metabolism. Structures were confirmed by NMR spectroscopy as those reported previously as animal metabolites (EFSA Scientific Report 2008, 140: 1-74).
Multi-pathway metabolites of epacadostat produced using microbial biotransformation
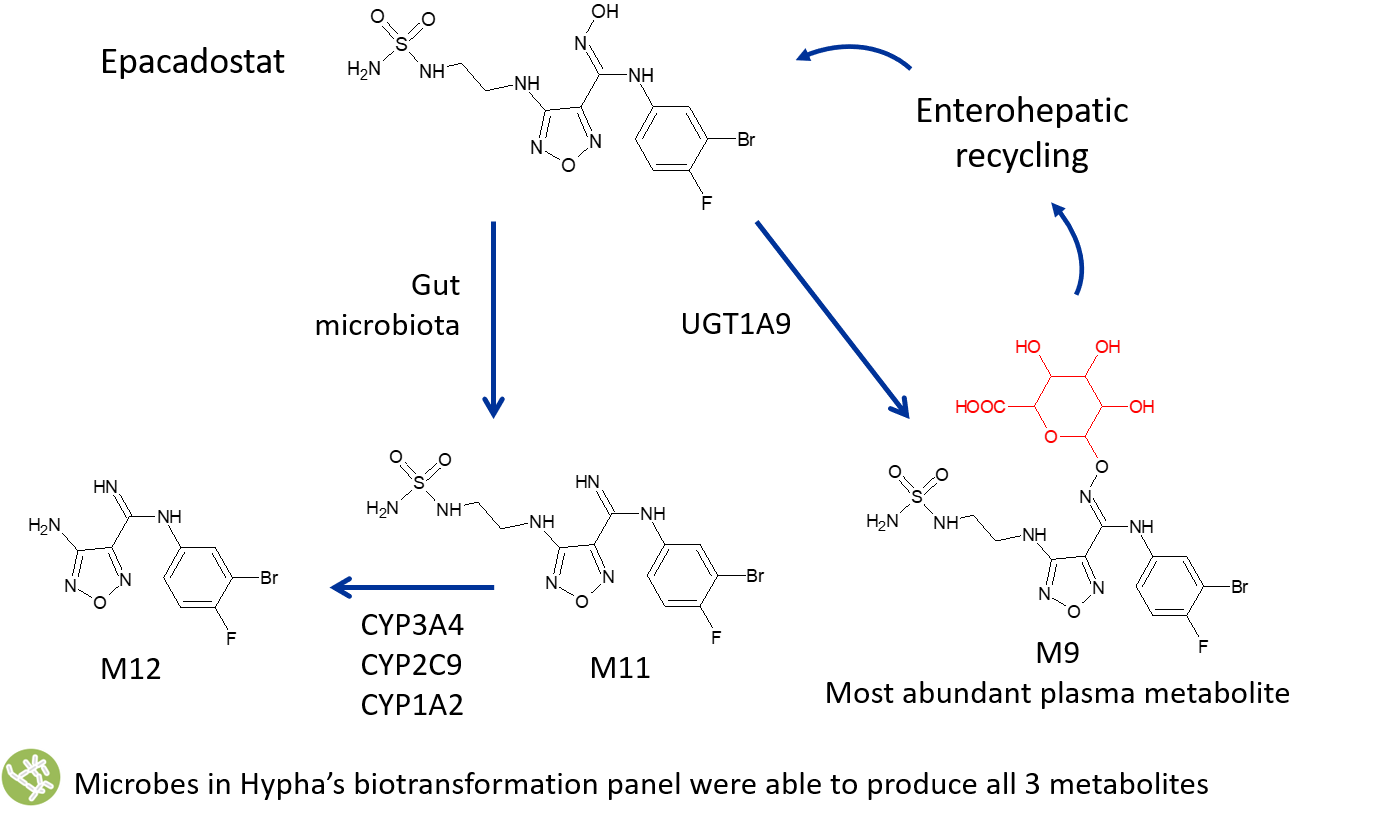
In vivo human metabolism of epacadostat (EPA) results in three major circulating metabolites from both primary and secondary pathways (Boer et al., 2016). Glucuronidation of EPA forms M9, the major metabolic pathway, in conjunction with the formation of an amidine M11 and an N-dealkylated metabolite, M12. EPA is metabolised by gut microbiota resulting in M11, which is absorbed and further modified by CYP enzymes to form the secondary metabolite M12.
Microbial biotransformation provided a route to access all three human metabolites, where different strains and dosing regimes were found to be optimal for the production of each metabolite. M12 was straightforward to synthesise chemically and thus was not accessed via microbial biotransformation. Scale-up of the most productive biotransforming strains for M9 and M11 enabled the supply of 112mg of the glucuronide and 69mg of the gut metabolite at 95% purity to Incyte.
The metabolites were used for structural confirmation and as analytical standards, as well as for further characterisation of metabolic pathways since M9 is deconjugated in the gut allowing for parent recirculation, and M12 is a product of M11.
Hydroxylation followed by sulfation using microbial biotransformation followed by chemical synthesis
In one client project, a sulfated metabolite was required which was produced in humans through an aryl hydroxylated intermediate. Hypha used microbial biotransformation to produce the intermediate metabolite followed by late-stage chemical sulfation to make and purify hundreds of milligrams of both the unlabelled and deuterated O-sulfated metabolite.
Multi-pathway metabolites of cenobamate made using both microbial biotransformation and chemical synthesis.
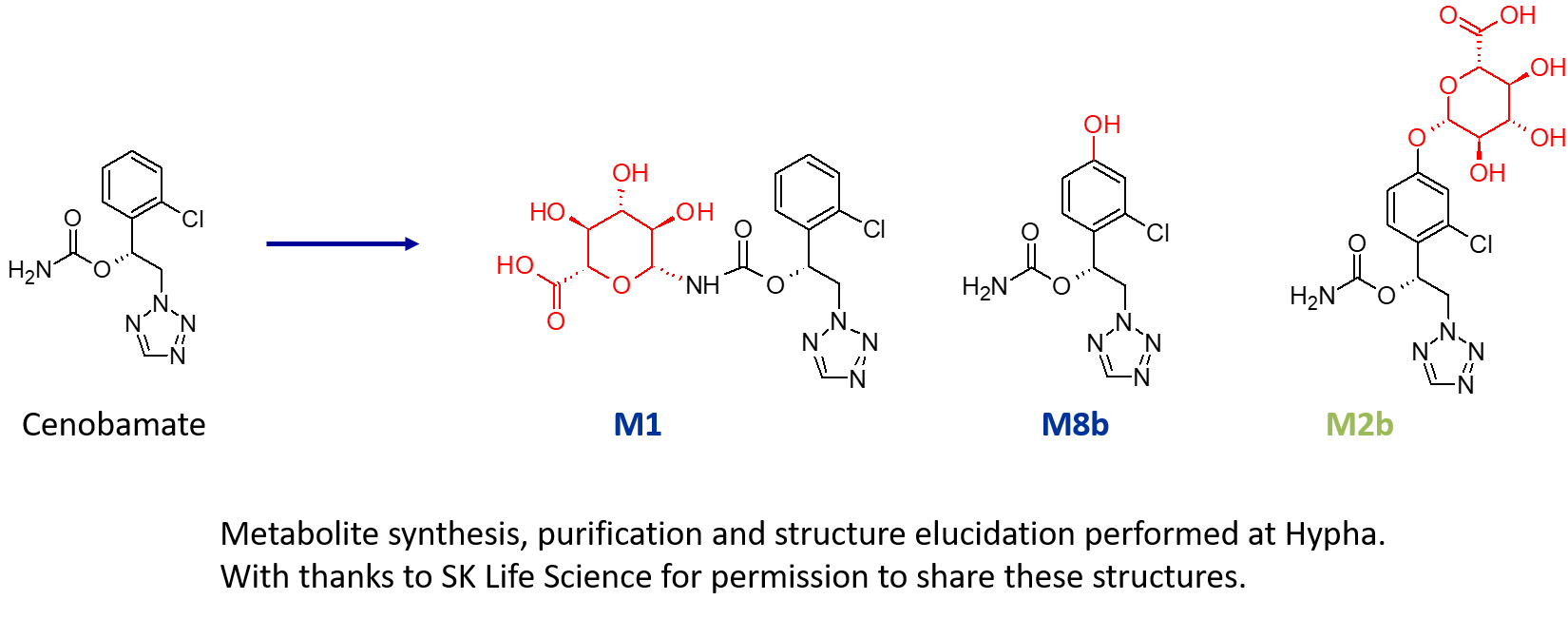
A US pharma client required > 200 mg of three metabolites of a drug; an N-glucuronide (M1), an indirect O-glucuronide (M2b) and a hydroxylated metabolite (M8b). As part of this project, multiple components of Hypha’s one-stop metabolite shop were employed, including chemical synthesis, microbial biotransformation as well as purification and structure elucidation by NMR.
M1, a major N-glucuronide, was accessed using chemical synthesis to make one gram. In addition to the N-glucuronide, an indirect O-glucuronide, M2b, and its aglycone, M8b, were also needed. To make M8b, the position of the hydroxyl group first had to be identified. To achieve this, Hypha chemists purified a small amount of M2b from human urine supplied by the client, and elucidated the structure of the conjugate using cryoprobe NMR spectroscopy. Then, knowing the position of hydroxylation from the structure of the phenolic glucuronide, 100s of mgs of M8b were synthesised. In order to access large amounts of M2b, a different approach was needed as this glucuronide was not amenable to chemical synthesis due to instability and formation of side products. Instead, M2b was successfully made through microbial biotransformation of the aglycone M8b. Following a screen to determine the best microbial catalyst, 560 mg of M8b was fed to one of our microbial strains, from which 254 mg of M2b was purified. All metabolites were supplied to SK Life Science with certificates of analysis.
Find out about synthesis of other metabolite types

Hypha has exceeded our expectations and is now a ‘go to’ lab for biosynthesis/synthesis/purification. Hypha’s team was a pleasure to work with and our complicated projects were handled with expertise and professionalism. Their excellent scientific communication and project data were extremely comprehensive and we received updates throughout the process. We will definitely be Hypha Discovery clients for life
Head of Toxicology/DMPK
US Pharma company
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
Metabolism of drugs often results in the formation of major circulating metabolites derived from mixed clearance pathways, and can include both primary and secondary metabolites. Human metabolism of Incyte’s investigational new drug epacadostat (EPA) forms 3 major circulating plasma metabolites (Boer et al., 2016). Glucuronidation of EPA to form M9 is the dominant metabolic pathway, in conjunction with formation of an amidine M11 and an N-dealkylated metabolite, M12. Boer and co-workers showed that reductive metabolism by gut microbiota results in M11, which is then absorbed and further modified by CYP enzymes to form the secondary metabolite M12.
Access to multiple metabolites needed to support clinical development is not always straightforward, and can sometimes mean that more than one technique needs to be applied to fulfil requirements. In one such project, a US pharma client required > 200 mg of three metabolites of a drug; an N-glucuronide (M1), an indirect O-glucuronide (M2b) and a hydroxylated metabolite (M8b). As part of this project, multiple components of Hypha’s one-stop metabolite shop were employed, including chemical synthesis, microbial biotransformation as well as purification and structure elucidation by NMR.
Metabolites of some agrochemical products such as pesticides and herbicides possess greater plant or mammalian toxicity compared to the parent compound, thus necessitating a need for their identification, study and provision of analytical reference standards to meet regulatory requirements. Where a synthetic route is challenging, or the identity of a metabolite is unknown, creation of metabolites by microbial biotransformation is often a successful alternative due to similarities of xenobiotic metabolism in mammals, birds, fish, soil, and to some extent, plants.1 Furthermore, biocatalysis affords aromatic and aliphatic site-selectivity as well as regiocontrol of aromatic hydroxylation.2
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Supplier Code of Conduct Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved.


