Metabolite Synthesis
Gut Metabolites
Gut Metabolites
Structural moieties of some drug compounds may be susceptible to chemical reaction in the gut environment, or to biotransformation by microbes in the intestine to gut-specific metabolites. This can be significant enough that resulting metabolites are absorbed and observed as circulating metabolites. Well known is the gut microbiota mediated biotransformation of ozanimod. Ozanimod and an intermediate oxidized metabolite undergo reductive cleavage and hydrolysis resulting in oxadiazole ring scission by anaerobic bacteria in the gut to generate a major metabolite in plasma.
Microbiota in the human gut are increasingly being considered as a virtual organ which plays a critical role in drug metabolism. The majority are obligate anaerobes from the genera Bacteriodes, Clostridium, Lactobacillus, Escherichia and Bifidobacteria, together with an assortment of other microorganisms [1]. The role of this “organ” in the biotransformation of drugs to form gut metabolites is complicated by variability in the individual composition of microbial species in the gastrointestinal tract. This potentially can cause differences in the drug response.
Direct microbiome-derived metabolism can lead to deactivation, reactivation through enterohepatic recycling, or biotransformation of a drug to a major metabolite. Metabolic reactions mediated by the microbiota that are known to significantly impact the biologic activity of drugs and xenobiotics involve reduction, hydrolysis, dihydroxylation, acetylation, deacetylation, proteolysis, deconjugation, and deglycosylation processes [2].
Hypha can monitor stability of drugs or their metabolites in a fecal environment. This is done over a time course comparing incubations in live and heat treated fecal samples and buffer, to check for reversion to the parent drug or further biotransformation. New metabolites can be isolated and their structures elucidated by NMR spectroscopy.
References
[1] Wilson ID and Nicholson JK, 2017. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 179, 204-222. doi: 10.1016/j.trsl.2016.08.002.
[2] Sousa et al., 2008. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm 363 (1-2), 1-25. doi: 10.1016/j.ijpharm.2008.07.009.
What We Do
Screening and scale-up service for MetID
Biotransformation of drugs using pooled human faecal material
Hypha’s human faecal pool can be used to screen for gut metabolites of drugs, as exemplified by the reduction of sulfasalazine and nizatidine. In these case studies, drugs were incubated in human faecal material derived from several mixed sex donors under oxygen depleted conditions. The reduced metabolites were easily detected by LC-MS.
Human faecal incubations can be scaled up to provide material for purification of metabolites prior to structure elucidation by cryoprobe NMR.
We have undertaken a number of projects for clients where identification of a suspected gut-derived metabolite of a drug in clinical trials was needed, sometimes revealing surprising structures. These have included reduced and unusual conjugated metabolites.
As well as gut microbiota-mediated metabolites, sometimes a conjugate can be formed in the complex matrix of faecal material, without direct involvement of enzymes produced by bacteria in the gut.

Key Features
Human origin faecal material
Pooled mixed sex source
Screen and scale-up
100s µg to mg amounts for MetID and biological testing
Stability testing
Time course assessment of parent drug and metabolites
Purification from complex mixtures
Our chemists are experts in isolating small amounts of metabolites from complex mixtures.
Structure elucidation by cryoprobe NMR
All data is interpreted and tabulated to publication standard.
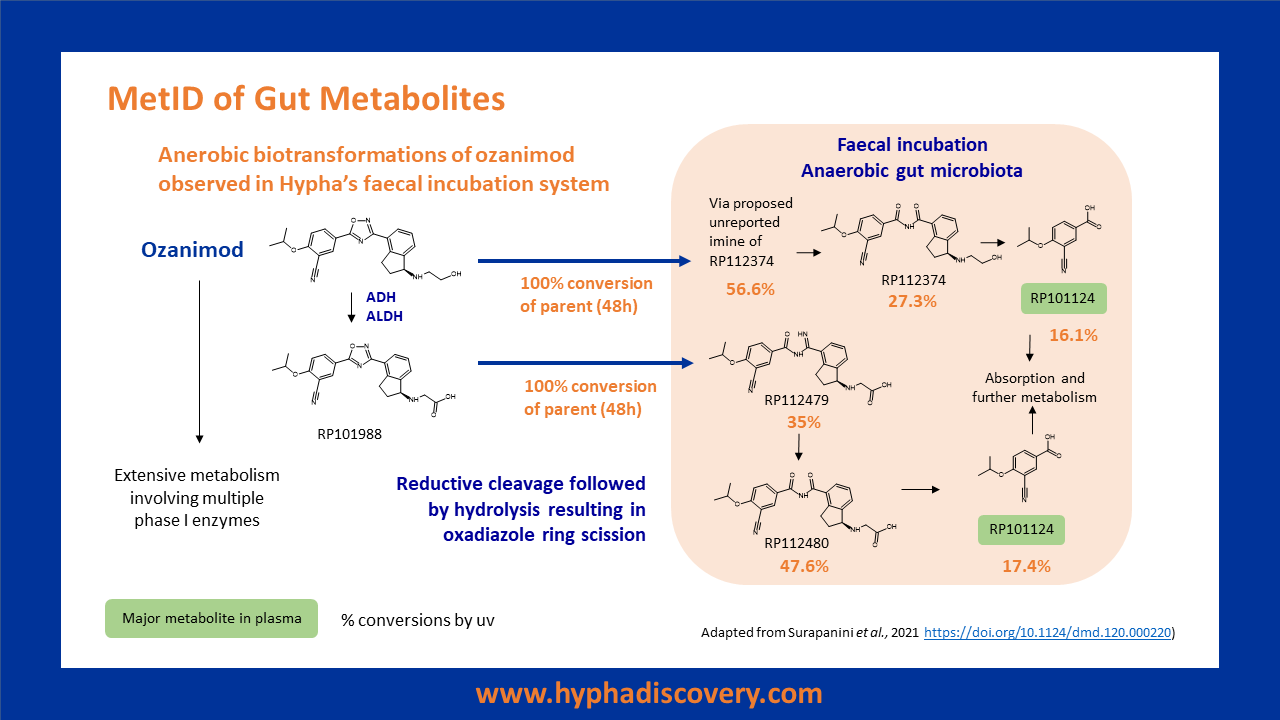
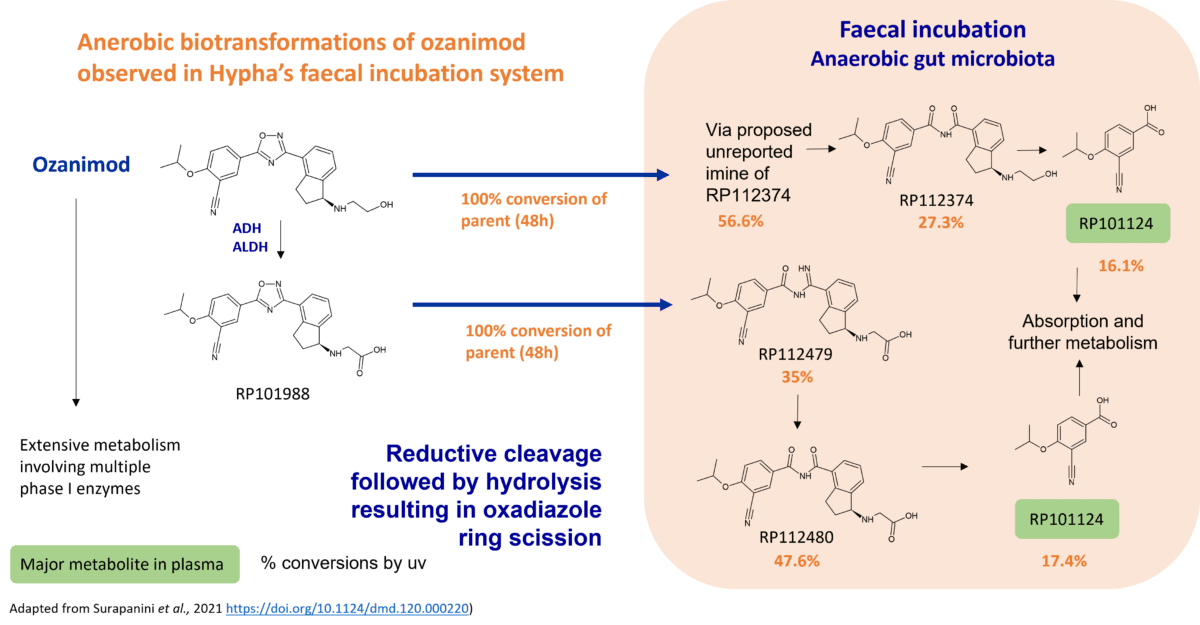
Case Study – faecal metabolism of ozanimod
Ozanimod undergoes extensive metabolism via multiple pathways, including biotransformations mediated by anaerobic bacteria in the gut.
Incubation of ozanimod and RP101988 (an ADH/ALDH metabolite of ozanimod) in Hypha’s human faecal incubation system under anaerobic conditions resulted in generation of all the reported metabolites shown in the scheme on the right. An unreported related metabolite was also observed.
Mechanisms involved reductive cleavage followed by hydrolysis resulting in oxadiazole ring scission.
Case study - production of a reduced metabolite of epacadostat
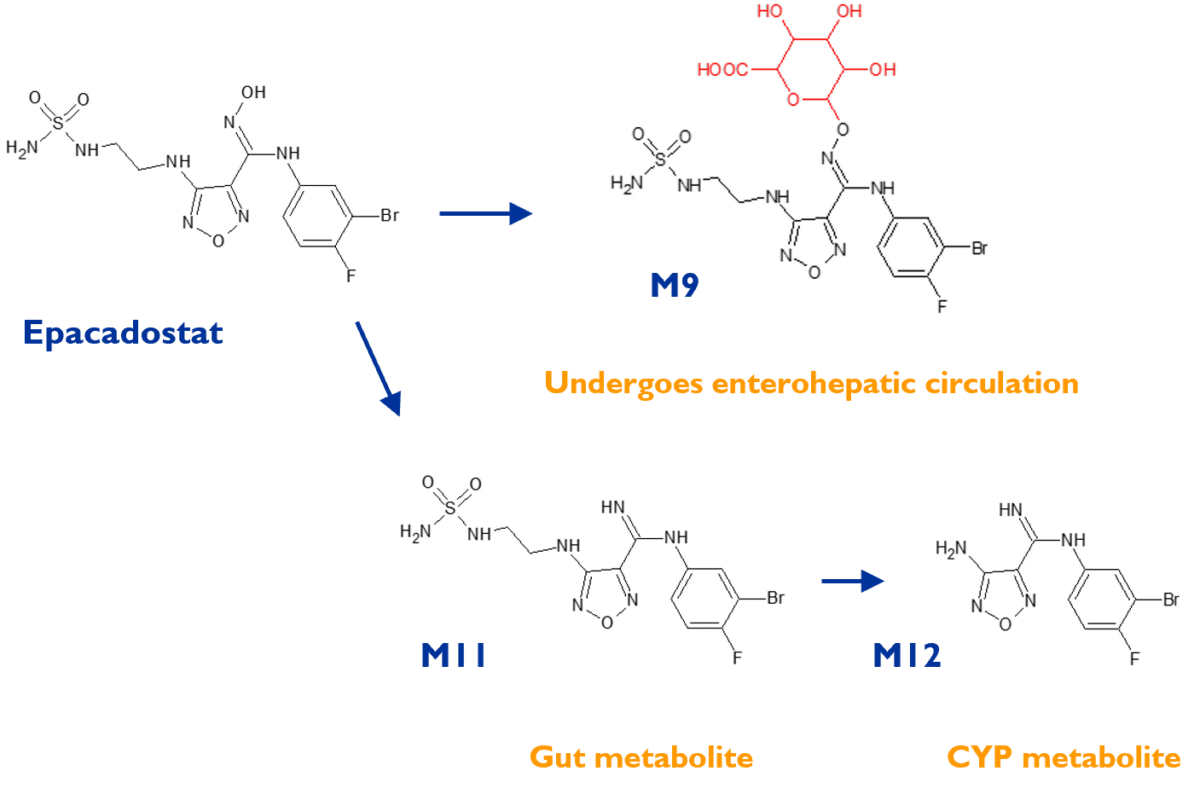
A reduced metabolite of epacadostat, M11, was surprisingly found to be produced by one of Hypha’s microbes under aerobic conditions. The metabolite was identical to that produced in a human faecal incubation. Microbial biotransformation of epacadostat also yielded a major O-glucuronide M9, and a metabolite M12 derived from CYP mediated metabolism of M11 (Boer at al.).
The O-glucuronide M9 is subject to enterohepatic recycling, involving hydrolysis of the glucuronide by gut bacteria enzymes. This is followed by the absorption of the drug back into systemic circulation, thereby leading to a prolonged elimination half-life and altered pharmacokinetics and pharmacodynamics.
Reference
Boer at al., 2016. Metabolism of Epacadostat in Humans. Drug Metabolism and Disposition 44 (10) 1668-1674; DOI: https://doi.org/10.1124/dmd.116.070680
Find out about synthesis of other metabolite types
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
Hypha’s human faecal pool can be used to screen for reduced metabolites of drugs, as exemplified with sulfasalazine and nizatidine.
In recent years, FDA guidance has advised initiating human metabolite profiling earlier in drug development, emphasizing the importance of metabolite identification and quantification to evaluate a drug metabolite’s safety and pharmacological activity. Praliciguat (IW-1973) is a soluble guanylate cyclase (sGC) stimulator in Phase 2 clinical trials for diabetic nephropathy and heart failure with preserved ejection fraction (HFpEF). During studies on metabolism of praliciguat in preclinical species and in human hepatocytes, a prominent direct O-glucuronide metabolite was detected.
Metabolism of drugs often results in the formation of major circulating metabolites derived from mixed clearance pathways, and can include both primary and secondary metabolites. Human metabolism of Incyte’s investigational new drug epacadostat (EPA) forms 3 major circulating plasma metabolites (Boer et al., 2016). Glucuronidation of EPA to form M9 is the dominant metabolic pathway, in conjunction with formation of an amidine M11 and an N-dealkylated metabolite, M12. Boer and co-workers showed that reductive metabolism by gut microbiota results in M11, which is then absorbed and further modified by CYP enzymes to form the secondary metabolite M12.

We have been very much impressed with the experience at Hypha Discovery. Hypha has provided us not only the services with high quality but also superior expertise in the field. From the study design, flow, production estimation to the timeline of the final product delivery, every single step was well planned. We were able to receive the high quality product in time. The communication was always quick and effective. There is no doubt that we will work with Hypha in the future when needed.
Yan Zhang, Senior Director DMPK
Nuvation Bio, USA
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk