Our Products
Recombinant Human Aldehyde Oxidase
Accessing Aldehyde Oxidase (AO) Metabolites
Human recombinant aldehyde oxidase (AOX1) is available for purchase by clients as a lyophilised crude enzyme powder for screening in client labs, and is also included in Hypha’s PolyCYPs+ Metabolite kit for accessing phase 1 oxidised metabolites. Both screening and scale-up of aldehyde oxidase reactions can be performed by the client themselves, or in the labs at Hypha.
Hypha’s aldehyde oxidase is a human recombinant enzyme expressed in E.coli. It has been tested against a number of common AO substrates to demonstrate oxidative catalytic activity. We have shown it is effective in metabolising known AO drug substrates such as zoniporide, carbazeran and phthalazine.
Aldehyde oxidase metabolites may also be accessed through microbial biotransformation using some of the microbes in Hypha’s biotransformation panels.
About Aldehyde Oxidase
Drug metabolism mediated by AO has become more prevalent as an oxidative clearance pathway in recently designed drugs derived from nitrogen-containing heterocycles. Further, mixed AO/P450 substrates may be subject to metabolic shunting, an important consideration during toxicology and DDI assessment of drugs which may require access to the metabolites in question.
Aldehyde oxidase is a cytosolic molybdoflavo enzyme with broad specificity which catalyses the oxidation of aromatic and aliphatic aldehydes and various nitrogen-containing heteroaromatic rings, as well as the reduction of several functional groups and hydrolysis of amides.
AO has profound species differences in expression and activity toward various substrates which is not always identified prior to clinical studies due to the lack of reliably predictive in vitro and in vivo models, which consequently can negatively impact on the development of several drug candidates. It is thus important to understand whether drug compounds are susceptible to aldehyde oxidase metabolism and identify any significant metabolites arising from this mechanism.
Case Studies
Screening for an oxidised metabolite of a drug candidate
In a recent client project conducted by Hypha on behalf of a client, a drug was screened against a number of PolyCYP enzymes, AOX1 and all five human FMOs. The structure was such that it would likely be susceptible to oxidation by AO, as well as other biotransformations. Several oxidised metabolites were produced by PolyCYPs isoforms; however, the target human metabolite was only observed to match those produced in the AO incubations, informing a path to chemical synthesis of the putative metabolite.
Provision of multiple human phase I metabolites via microbial biotransformation
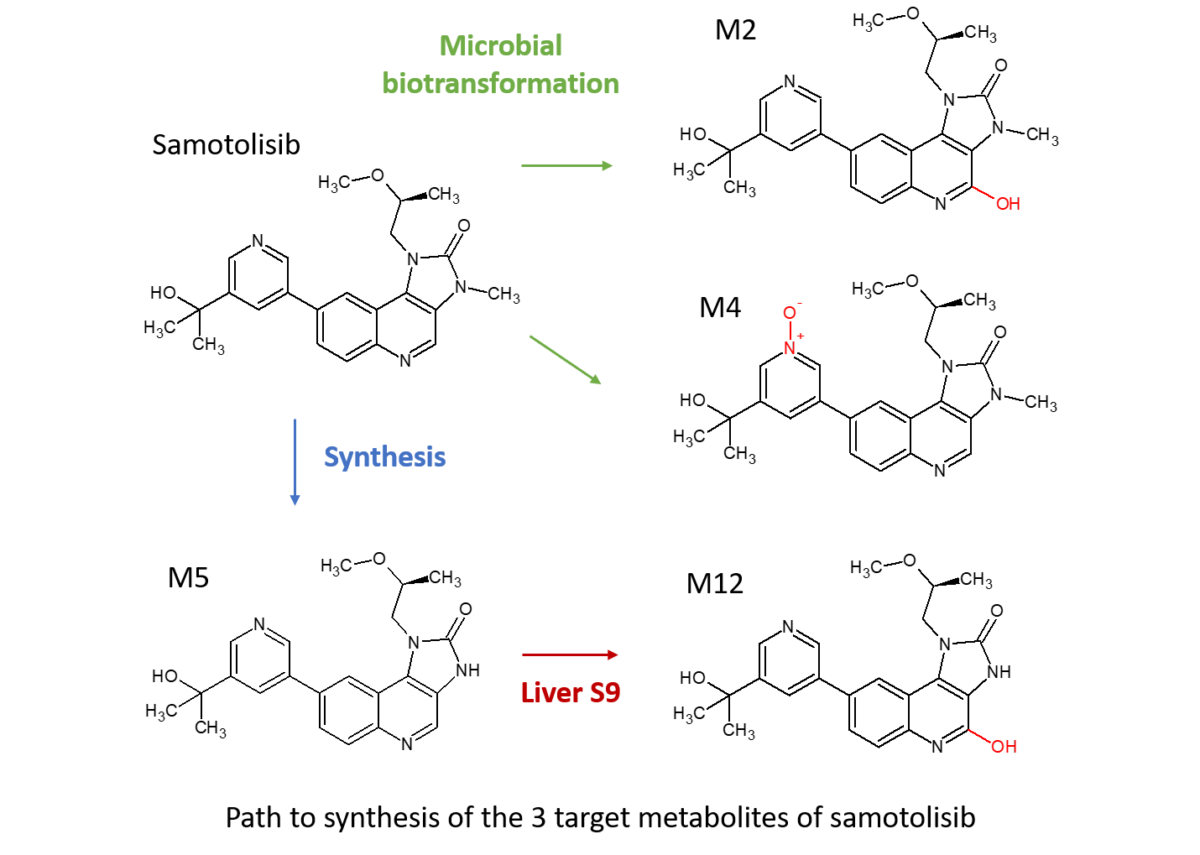
Zhou and colleagues at Lilly presented a poster at the 2018 ISSX meeting in Montreal on “Elimination of [14C]-LY3023414 by Aldehyde Oxidase and CYP Enzymes in Humans Following Oral Administration.” Both AO and CYP enzymes were responsible for the metabolic clearance of LY3023414 (samotolisib) with the non-CYP enzymes mediating approximately half of the clearance of the drug. The predominant metabolic clearance pathways were aromatic hydroxylation of the quinoline moiety (M2), N-demethylation (M5) and quinoline oxidation with N-demethylation (M12).
No metabolism was observed when tested vs 5 human recombinant CYPs, however screening of LY3023414 against a subset of Hypha’s biotransforming strains generated a number of metabolites. The best microbial strain was scaled-up to 6L to access the AO mediated hydroxylated metabolite (M2, 20.1mg) and an N-oxide (M4, 66.3mg) . Subsequent incubation of the synthesised CYP intermediate M5 vs Cyno S9 enabled production of a CYP/AO mediated metabolite (M12, 18.4mg). Metabolites were purified to >95% purity by Hypha and the structures confirmed by LC-MS and NMR.
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
There has been a notable increase in metabolism of new drug candidates through non-CYP phase I pathways such as those mediated via aldehyde oxidase (AO).1 Further, mixed AO/P450 substrates may be subject to metabolic shunting, an important consideration during toxicology and DDI assessment of these drugs.2 Access to metabolites may thus be important to consider for drugs with mixed metabolism.
In this paper, authors from Hypha and Incyte Corporation discuss the impact and application of biotransformation of drugs by mammalian systems, microorganisms, and recombinant enzymes, covering active and reactive metabolites, the impact of the gut microbiome on metabolism, and how insights gained from biotransformation studies can influence drug design.
In Chapter 4 of the book on “Identification and quantification of drugs, metabolites, drug metabolizing enzymes and transporters”, Hypha authors summarise the different methods employed for producing metabolites of drugs, illustrated with representative examples from the literature and work undertaken at Hypha. The chapter also includes a discussion and examples of the use of NMR spectroscopy for structure elucidation of metabolites.
See our other solutions for metabolite synthesis
Hypha’s One-Stop Metabolite Shop enables synthesis, purification and characterization of all the main types of mammalian phase 1 and 2 metabolites.
Chemical Synthesis
Late-stage chemical glucuronidation
Mammalian biotransformation
Panels of liver S9s / microsomes
Recombinant enzymes
PolyCYPs, hrCYPs, AOX, FMOs
Microbial biotransformation
Proven panels of bacteria and fungi
Purification & structure elucidation
COAs, acquisition / interpretation of NMR data

“Hypha Discovery has been a valuable metabolite ID partner. Hypha have provided biotransformation, metabolite purification and structure elucidation answers to some of our most challenging metabolism and metabolite ID problems. We really appreciate the breadth of expertise available at Hypha Discovery and will definitely reach out for future work.”
Director of DMPK
US Pharma Company
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk