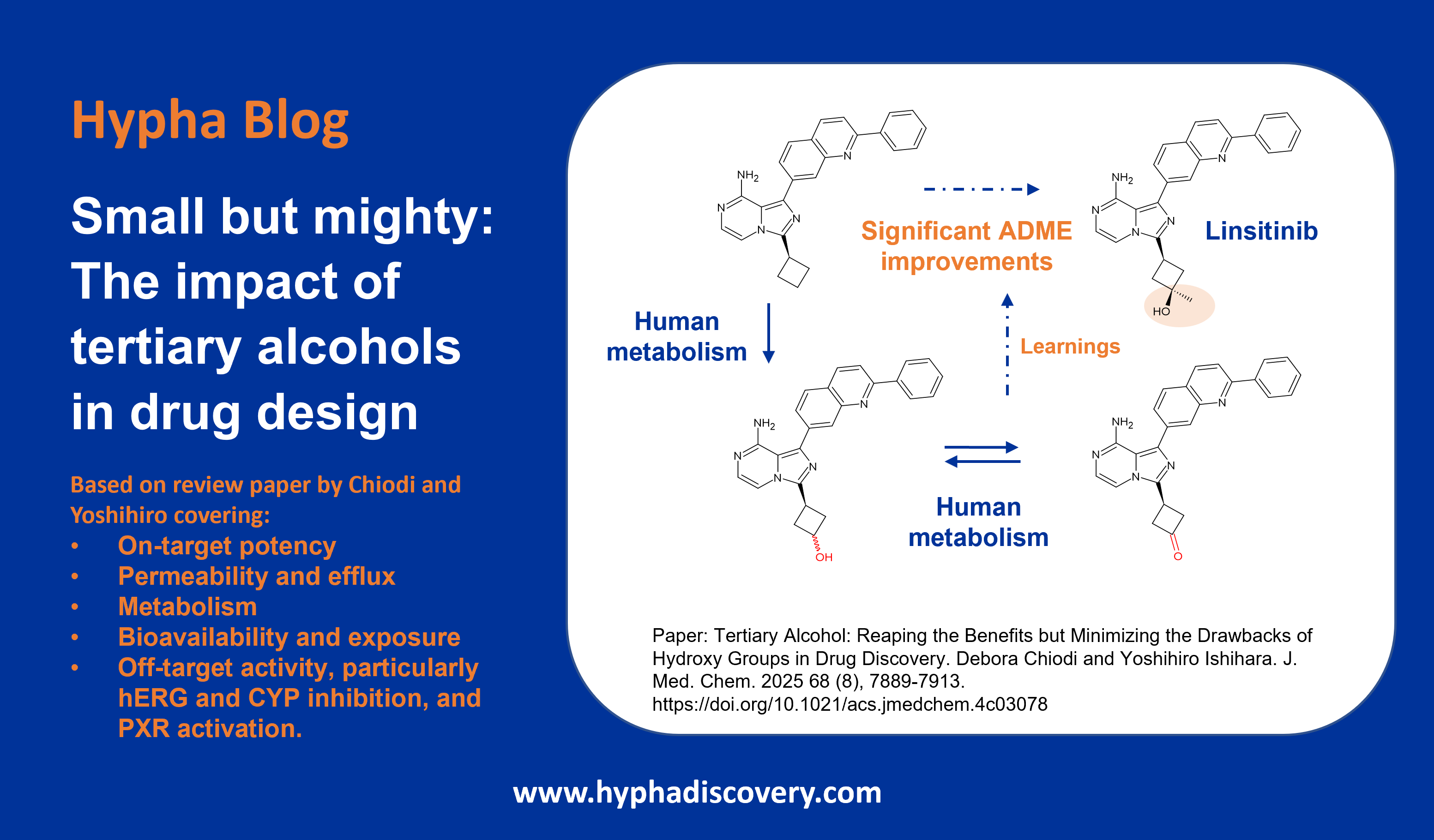
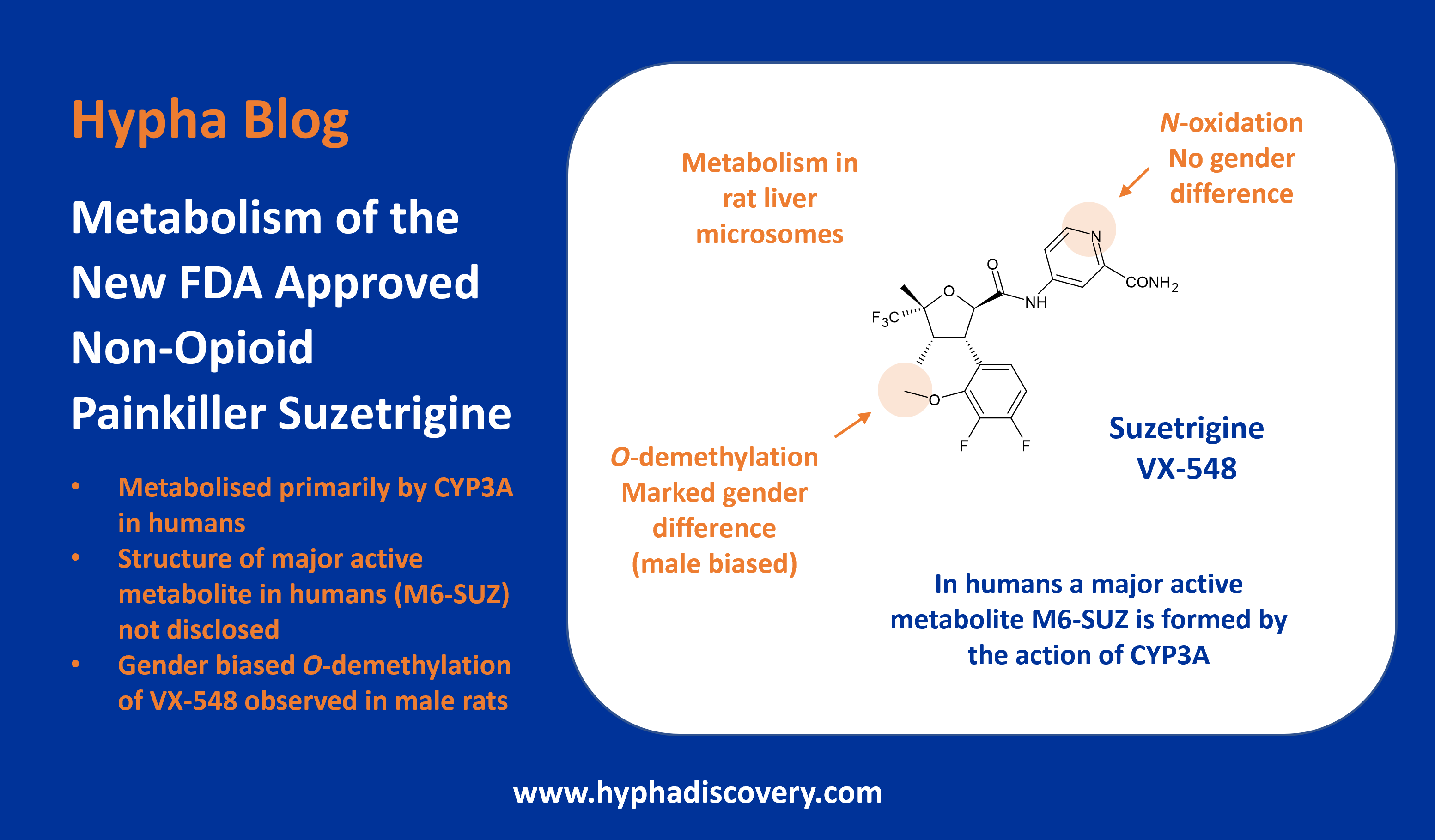
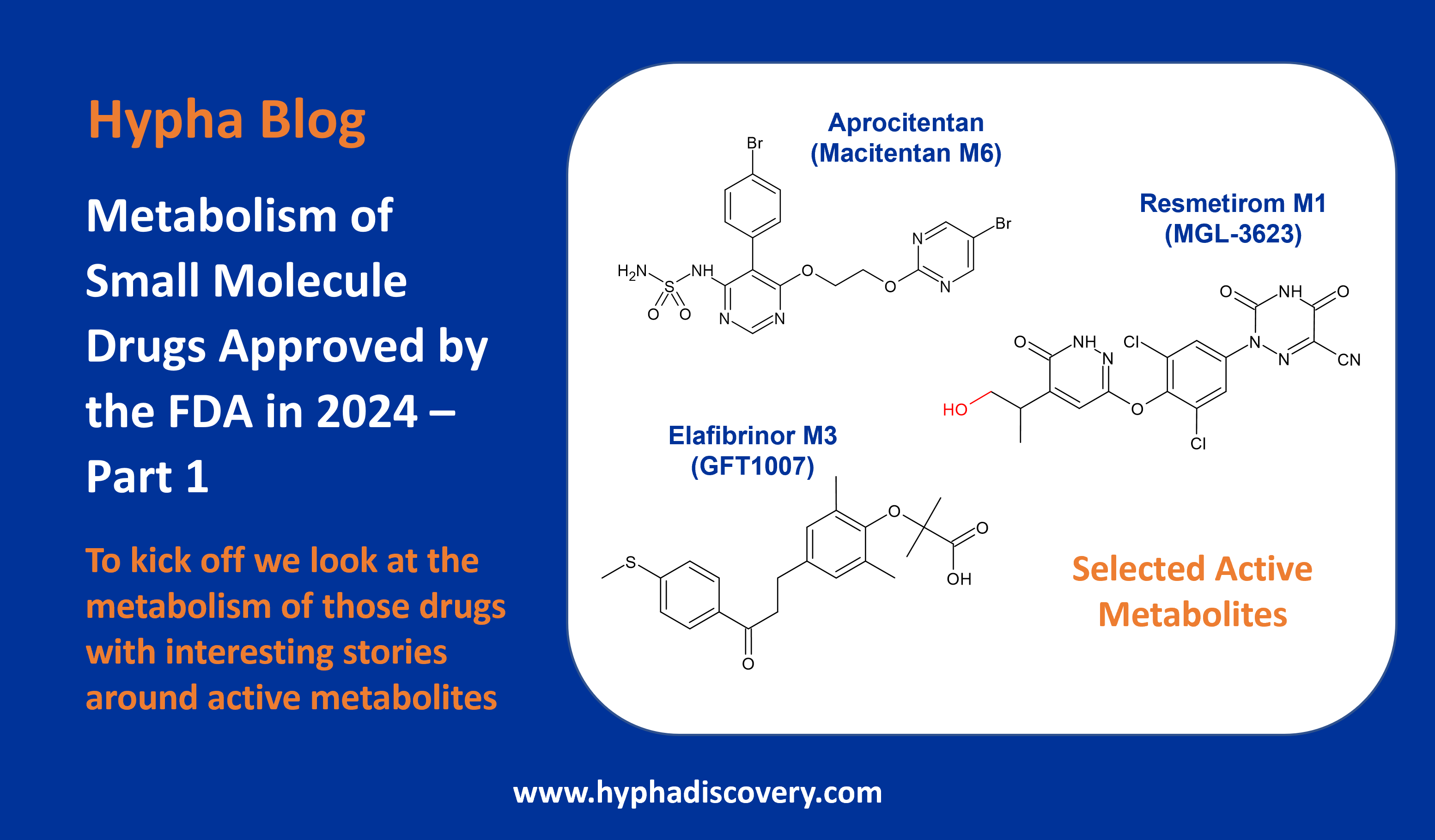
Metabolism of 2023 FDA Approved Small Molecules – PART 1
By Julia Shanu-Wilson
2023 was a fruitful year for new drugs, with a crop of 34 small molecules out of a total of 55 new drugs approved by the FDA [1]. Over the next couple of months we’ll be taking a look at the metabolism of these small molecule drugs, starting with those where there is mention of the involvement of active metabolites. This constitutes an interesting subset of 9 drugs, corresponding to over 25% of the new small molecule drugs approved in 2023!
The bigger picture
An analysis of the metabolism of the most commonly prescribed drugs in 2022 [2] revealed that of the 89 subject to drug metabolism, 75% were cleared via phase I mechanisms, with nearly half of these metabolised by CYP3A4.
In comparison, of the 28 (82%) small molecule drugs approved by the FDA in 2023 that were subject to drug metabolism, 64% were metabolised by phase I routes with 39% primarily via CYP3A4. Interestingly, there are a number of drugs biotransformed by multiple enzymes i.e. with no one route dominating, or involving multiple cytochrome P450 enzymes (CYPs). Major contributing non-phase I routes were due to glucuronidation by uridine 5′-diphospho-glucuronosyltransferases (UGTs). Where data was publicly available, the routes of metabolism for each drug is listed in Table 1 in the Appendix at the end of this article.

Figure 1: Comparison of the major routes of metabolism of small molecule drugs approved by the FDA in 2023 and 2022
Not surprisingly, active metabolites of 8 of the subset of 9 drugs listed in Table 2 were generated through phase I mechanisms, largely through the action of CYPs. Enzymes involved include CYP1A2, CYP2C8, CYP3A4, CYP4F2, and aldehyde oxidase (AOX).

Table 2: Small molecule drugs approved by the FDA in 2023 with reported or published active metabolites
Stereoisomers uncovered
Daprodustat undergoes oxidation almost exclusively (95% contribution) by CYP2C8 to 6 metabolites which constitute 60% of total circulating radioactivity in the human radiolabelling study [3]. Of these 6 metabolites, M2, M3 and M13 each account for more than 10% of circulating radioactivity in plasma (i.e. are major metabolites according the FDA Metabolites in Safety Testing guidelines). To complicate matters, 5 of the 6 oxidative metabolites exist in different stereoisomeric forms, resulting in 14 separate species, revealed from development of a chiral method involving the use of supercritical fluid chromatography (SFC) to separate this complex mixture [4].
In vitro and non-clinical studies suggest that these identified metabolites have a comparable pharmacological activity to the parent drug and may contribute to the response in vivo; however, the extent of the pharmacological contribution of each metabolite has not been investigated or reported [3].
Due to the predominant clearance of the drug by CYP2C8, daprodustat is contraindicated in patients taking strong CYP2C8 inhibitors such as gemfibrozil [3].

Figure 2: Active hydroxylated metabolites of daprodustat
FDA request for additional studies

Fezolinetant is primarily metabolized by CYP1A2, with a major circulating metabolite, ES259564 observed in plasma. Despite the metabolite being 20-fold less potent at the target receptor than the parent drug [5], the FDA was originally concerned that due to the exposure of the metabolite being 2-fold higher than that of the parent, the accumulation of the metabolite might have a clinical impact. This resulted in a request to conduct additional studies with ES259564 prior to the NDA submission. The metabolite was duly profiled and shown to contribute only 5% of the overall pharmacological activity and had no off-target activities [6]. A deuterated version of fezolinetant has been patented which was shown to provide an improved CYP profile and enabled improvement in extending the in vivo half-life of the drug [8].
Since fezolinetant is excreted in urine, the AUC (area under the curve) of ES259564 increases by approximately 380% in women with severe renal failure. The drug is therefore contraindicated in cases where severe renal impairment or end‐stage renal disease has been confirmed, as well as in combination with CYP1A2 inhibitors [7].
Patented active metabolites
Gepirone’s metabolites have been patented for “the manufacture of a medicament for the treatment of psychological disorders” [9]. The drug is metabolised by CYP3A4 to 2 major active metabolites; 1- (2-pyrimidinyl)-piperazine (1-PP) and 3-OH-gepirone [10]. These and another active metabolite, 3,5-dihydroxy gepirone, were patented due to their superior bioavailability, faster onset of action, and more stable plasma levels when administered in vivo compared to other azapirones. In fact, 3-OH-gepirone was found to be highly selective for the 5-HT1A receptor among the serotonergic subtypes and is more selective than the parent drug in having only very weak binding affinity at dopaminergic and α-adrenergic receptors. Additionally, in the patent it was reported that the improved water solubility of 3-OH-gepirone (and other bioactive gepirone metabolites) reduces first-pass degradation in the liver whilst retaining sufficient lipid solubility needed for psychoactive compounds that may interact within receptors in the brain [9].

Figure 3: Active metabolites of gepirone
Involvement of aldehyde oxidase
Momelotinib has a long history, passaging through many companies before finding its fifth and final home at GSK with approval for treatment of myelofibrosis in adults with anaemia. Its metabolism involves multiple mechanisms including oxidation and scission of the morpholine ring, amide hydrolysis, N-dealkylation, nitrile hydrolysis, nitrile oxidation, and glucuronidation [11]. Work unravelling its biotransformation was performed by scientists at Gilead where they showed that M21, a morpholino lactam metabolite, circulated at 64.2% of [14C] AUC in pooled human plasma. Interestingly another metabolite resulting from amide hydrolysis, M19, dominated in rat and dog. M19 is pharmacologically inactive.
Multiple CYPs were shown to be involved in metabolism of momelotinib including CYP3A4, 2C8, 2C19, 2C9 and 1A2. Due to the advantageous involvement of multiple CYPs, the PK of momelotinib is proposed to not be altered in a clinically meaningful way by an inhibitor or inducer of any single enzyme [11]. However, CYPs are only involved in the first step in formation of M21, bioactivating the morpholine ring to a carbinolamine intermediate, which is then followed by a second oxidation to M21 by aldehyde oxidase. The increased formation of M21 in humans compared with nonclinical species was consistent with rats having lower AOX activity and dogs being devoid of such activity. Given the disproportionately higher M21 human systemic AUC exposure, coadministration of both the parent drug and M21 was undertaken to increase M21 systemic AUC exposure in rats to meet regulatory safety testing guidelines [11].

Figure 4: Major routes of metabolism of momelotinib
Although M21 was 2- to 3-fold less active than the parent drug for inhibition of JAK1/2 (Janus protein tyrosine kinases) and activin A receptor type 1 (ACVR1) in vitro, the calculated pharmacologic activity index (PAI) suggests it contributes significantly to the pharmacological activity. This is based on PAI values of 47% (JAK1/2 inhibition) and 57% (ACVR1 inhibition), above the >25% threshold indicative of a pharmacologically active metabolite [11]. It is interesting to note that M21 and the parent drug are potent inhibitors of both JAK1/2 and ACVR1, significant because inhibition of ACVR1 suppresses liver hepcidin expression and restores iron homeostasis and erythropoiesis, thus countering the anaemia which is a hallmark of myelofibrosis [12].
Pyrrolidinone ring hydroxylation
Nirmatrelvir (+ ritonavir) is probably the most well-known drug approval of last year, due it its potential impact on the Covid-19 pandemic. It is well known that the co-administration with ritonavir was designed to put a brake on the rapid metabolism of the drug by inhibiting CYP3A4, the main CYP responsible.
CYP3A4 catalyses the formation of prominent metabolites from oxidations at 3 different moieties in nirmatrelvir; the pyrrolidinone ring, 6,6-dimethyl-3-azabicyclo[3.1.0]hexane, and the tertiary-butyl group. It is hydroxylation of the pyrrolidinone ring which results in a major active metabolite M4. M4 possesses in vitro nanomolar activity against recombinant SARSCoV-2 3CLpro similar to the parent drug, and represses SARS-CoV-2 replication in epithelial Vero E6 Cells. However, the corresponding EC50 for M4 in the cellular antiviral assay was relatively weaker than the one observed with the parent drug (690 nM vs 74.5 nM) [13].

Figure 5: Biotransformation of nirmatrelvir to hydroxylated metabolites
Active keto metabolites
Palovarotene is another drug extensively metabolised by CYP3A4, resulting in the formation of 5 metabolites – M1 (6,7-dihydroxy), M2 (6-hydroxy), M3 (7-hydroxy), M4a (6-oxo), and M4b (7-oxo). In the human radiolabelled ADME study, M2, M3, M4a and M4b collectively represented 40% of the total exposure in plasma. Using an in vitro transactivation assay, the activity of metabolites M2, M3, M4a, and M4b contributed respectively 2%, 7%, 23%, and 12% of the parent drug, with the secondary keto metabolites contributing more activity than their corresponding hydroxylated precursors [14].

Figure 6: Active metabolites of palovarotine
Selective activity in vivo
 Quizartinib is similarly extensively biotransformed by CYP3A4/5 to multiple metabolites including a major circulating metabolite M24 (AC886). Reactions involve oxidation, reduction, dealkylation, deamination, hydrolysis and combinations thereof [15,16]. M24 was equally as selective FLT3 inhibitor as the parent drug, binding fewer kinases with high affinity than other FLT3 inhibitors evaluated, and possessing high affinity for the FLT3 kinase. In addition to having a similar kinase binding profile, M24 reduced the cell viability of FLT3-ITD cells to the same degree as quizartinib, and each independently displayed antitumor activity in a mouse xenograft model [17]. Both quizartinib and AC886 affect cardiac ion channels; at 3 μM both statistically significantly inhibited hERG currents by 16.4% and 12.0%, respectively [15].
Quizartinib is similarly extensively biotransformed by CYP3A4/5 to multiple metabolites including a major circulating metabolite M24 (AC886). Reactions involve oxidation, reduction, dealkylation, deamination, hydrolysis and combinations thereof [15,16]. M24 was equally as selective FLT3 inhibitor as the parent drug, binding fewer kinases with high affinity than other FLT3 inhibitors evaluated, and possessing high affinity for the FLT3 kinase. In addition to having a similar kinase binding profile, M24 reduced the cell viability of FLT3-ITD cells to the same degree as quizartinib, and each independently displayed antitumor activity in a mouse xenograft model [17]. Both quizartinib and AC886 affect cardiac ion channels; at 3 μM both statistically significantly inhibited hERG currents by 16.4% and 12.0%, respectively [15].
The final two drugs with active metabolites involve rather more atypical drug metabolism pathways. Taurolidine is metabolised rapidly via the metabolites taurultam and methylol taurinamide, which also have a bactericidal action, to taurine [18]. The peptide drug zilucoplan is expected to be degraded into small peptides and amino acids via catabolic pathways, however there are 2 circulating plasma metabolites (RA103488 and RA102758) observed at approximately 10% of the parent AUC. Interestingly RA103488 is formed by ω-hydroxylation of the C16 lipid tail by CYP4F2 and has pharmacological activity similar to zilucoplan, However, since it is present at a much lower concentration compared to the parent drug the contribution of RA103488 to pharmacological activity is predicted to be low. The other metabolite RA102758 is formed by protease mediated degradation and is pharmacologically inactive [19].
In part 2 we’ll be looking at some interesting biotransformations of other small molecule drugs approved by the FDA in 2023, including involvement of glucuronidation.
Note that where Hypha may have been involved in any of the projects described, no details on metabolites other than what is publicly available have been disclosed in this article.
References
[1] 2023 Novel Small Molecule FDA Drug Approvals. https://drughunter.com/articles/2023-novel-small-molecule-fda-drug-approvals/).
[2] Iversen et al., 2022. Drug metabolism and drug transport of the 100 most prescribed oral drugs. Basic Clin Pharmacol Toxicol. 131(5): 311- 324. https://doi.org/10.1111/bcpt.13780
[3] FDA prescribing information for daprodustat. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216951s000lbl.pdf
[4] Licea Perez et al., 2016. Overcoming bioanalytical challenges associated with the separation and quantitation of GSK1278863, a HIF-prolyl hydroxylase inhibitor, and its 14 stereoisomeric metabolites. J Chromatogr B Analyt Technol Biomed Life Sci. 1009-1010: 7-16. https://doi.org/10.1016/j.jchromb.2015.11.057
[5] FDA prescribing information for fezolinetant. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216578s000lbl.pdf
[6] NDA 216578- ESN364 (fezolinetant) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/216578Orig1s000MedR.pdf
[7] Shaukat et al., 2023. Veozah (Fezolinetant): A Promising Non-Hormonal Treatment for Vasomotor Symptoms in Menopause. Health Sci Rep. 6(10):e1610. https://doi.org/10.1002/hsr2.1610
[8] Patent EP3428168A1. https://patents.google.com/patent/EP3428168A1/en
[9] Patent EP1242061B1. https://patents.google.com/patent/EP1242061B1/en
[10] FDA prescribing information for gepirone. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/021164s000lbl.pdf
[11] Zheng et al., 2018. Pharmacokinetics and Disposition of Momelotinib Revealed a Disproportionate Human Metabolite—Resolution for Clinical Development. Drug Metabolism and Disposition 46 (3), 237-247; DOI: https://doi.org/10.1124/dmd.117.078899
[12] Asshoff et al., 2017. Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood 129(13): 1823-1830. https://doi.org/10.1182/blood-2016-09-740092
[13] Eng et al., 2022. Pharmacokinetics and Metabolism of Nirmatrelvir. Drug Metabolism and Disposition 50 (5) 576-590; DOI: https://doi.org/10.1124/dmd.121.000801
[14] FDA prescribing information for palovarotene. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215559s000lbl.pdf
[15] FDA prescribing information for quizartinib. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216993s000lbl.pdf
[16] Sanga et al., 2017. An open-label, single-dose, phase 1 study of the absorption, metabolism and excretion of quizartinib, a highly selective and potent FLT3 tyrosine kinase inhibitor, in healthy male subjects, for the treatment of acute myeloid leukemia. Xenobiotica, 47:10, 856-869. https://doi.org/10.1080/00498254.2016.1217100
[17] Aikawa et al., 2020. Quizartinib, a selective FLT3 inhibitor, maintains antileukemic activity in preclinical models of RAS-mediated midostaurin-resistant acute myeloid leukemia cells. Oncotarget. 11: 943-955. Retrieved from https://www.oncotarget.com/article/27489/text/
[18] William et al., 1995. Taurolidine, an antilipopolysaccharide agent, has immunoregulatory properties that are mediated by the amino acid taurine. Journal of Leukocyte Biology, 58 (3), 299-306. https://doi.org/10.1002/jlb.58.3.299
[19] FDA prescribing information for zilucoplan. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216834s000lbl.pdf
Appendix – Table 1: Summary of metabolism of small molecule drugs approved by the FDA in 2023