API Degradation Products
An Oxidation Toolkit
Hypha have put together a set of late-stage chemical oxidation conditions, resulting in a toolbox for the synthesis and identification of API degradation products. Biotransformation tools and the isolation of multiple products from complex mixtures, widens the suite of techniques to produce and identify degradation products.
Oxidative degradation pathways commonly result in the most complex reaction profiles, highlighted by Nanda and colleagues who state that, “…faster and practical ways of enriching an API with relevant oxidative degradates and their isolation in pure form is highly desirable.” Thus, availability of an oxidation toolkit incorporating broad chemistry, is beneficial for rapidly generating oxidised products.
Chemical Oxidation Screen
A chemical oxidation screen has been developed which creates oxidised products using 14 diverse conditions. Broad oxidation chemistry has been incorporated through a comprehensive evaluation of the literature and in-house knowledge, with the inclusion of both peroxide and radical reagents. The screen has been validated with a set of structural diverse substrates.
Following a quick optimization step, reactions can be scaled to make gram quantities of the degradate. Multiple products can be isolated from complex mixtures and structures elucidated using cryoprobe NMR.
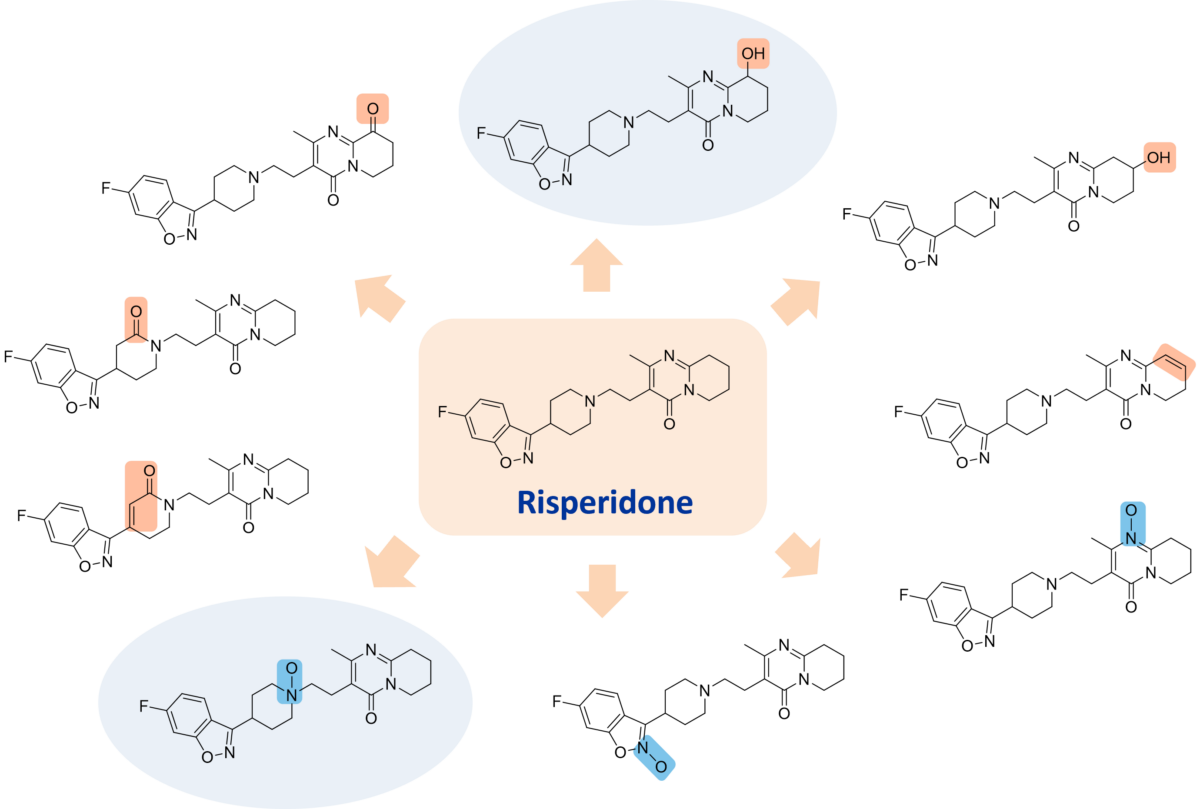
Case Study – Chemical Oxidation of Risperidone
Risperidone was screened against a diverse set of oxidation conditions and the products analysed by LC-MS. Reactions in which oxidation products were observed were scaled up resulting in the isolation of 10 different oxidation products. Structures were elucidated through use of cryoprobe NMR.
The two major degradants observed for risperidone are an N-oxide and 9-hydroxy risperidone (also known as paliperidone, the primary active metabolite of rispiridone, sold at Invega).
Both the N-oxide and 9-hydroxy risperidone major degradation products were produced (shaded) along with 8 other oxidised derivatives.
References
Nanda et al., 2019. Enrichment of Relevant Oxidative Degradation Products in Pharmaceuticals With Targeted Chemoselective Oxidation. Journal of Pharmaceutical Sciences, 108, 4, 1466-1475.
Tomar et al., 2004. Identification and characterization of major degradation products of risperidone in bulk drug and pharmaceutical dosage forms. Journal of Pharmaceutical and Biomedical Analysis, 36, (1), 231-235