Synthesis of deuterated metabolites
Both oxidised and conjugated metabolites can be produced using either biotransformation or late-stage chemical synthesis from the deuterated parent compound.
Case study using a biotransformation approach
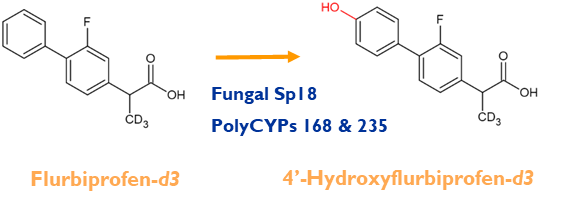
The non-steroidal anti-inflammatory flurbiprofen is subject to both CYP and UGT mediated metabolism. In humans CYP2C9 is responsible for the major metabolite, 4’-hydroxyflurbiprofen.
Screening of flurbiprofen against a panel of PolyCYPs® enzymes and microbes resulted in several products. A fungal biotransformation produced the major metabolite 4’-hydroxyflurbiprofen with almost complete conversion. The correct product was confirmed by NMR spectroscopy of the purified metabolite.
Metabolism of flurbiprofen-d3 also results in hydroxylation at the same position by the same enzymes and microbes, albeit the fungal biotransformation proceeding more slowly than observed with the non-deuterated drug. It is expected that biotransformation processes may be affected by deuteration at sites of metabolism in compounds.
Example client projects
non-steroidal anti-inflammatory flurbiprofen is subject to both CYP and UGT mediated metabolism. In humans CYP2C9 is responsible for the major metabolite, 4’-hydroxyflurbiprofen.
Hydroxylated O-sulfate
Sometimes both biotransformation and chemical synthesis routes are utilised in order to make a metabolite. This tandem approach is especially useful for multi-step metabolites. In one example, 100s of milligrams of both unlabelled and deuterated analogues of an O-sulfated metabolite were produced using these methods in series. The parent drug was subject to multiple routes of metabolism with the major route involving oxidation followed by sulfation. Microbial biotransformation was used to make the correct isomer of the aryl hydroxylated intermediate. Following purification of the intermediate metabolite, chemical synthesis was used to make 430 mg of an O-sulfated metabolite observed in human liver microsomes at a purity of >99% by LC-UV-ELSD.
Acyl glucuronide
Sometimes both biotransformation and chemical synthesis routes
are utilised in order to make a metabolite. This tandem approach is especially
useful for multi-step metabolites. In one example, 100s of milligrams of both
unlabelled and deuterated analogues of an O-sulfated metabolite were produced
using these methods in series. The parent drug was subject to multiple routes of metabolism with the major route involving oxidation followed by sulfation.
Microbial biotransformation was used to make the correct isomer of the aryl hydroxylated intermediate. Following purification of the intermediate metabolite, chemical synthesis was used to make 430 mg of an O-sulfated
metabolite observed in human liver microsomes at a purity of >99%
N-glucuronide
This project necessitated transitioning from a human liver S9 biotransformation to a more scalable late-stage chemical synthesis method. Following a quick optimisation, gram amounts of a d7 N-glucuronide was supplied to the client at >99% purity by LC-UV-ELSD and 97% purity by qNMR analysis