Poster presented at: Drug Discovery Chemistry 2023, San Diego, USA
Structural Identification and Synthesis of the Acyl Glucuronide of the Macrolide Mcl-1 Inhibitor AZD5991
Abstract
AZD5991 is a potent and selective inhibitor of Mcl-1 which entered clinical development for treatment of haematological malignancies1. Preclinical metabolite profiling of AZD5991 in human hepatocytes revealed the presence of an abundant glucuronide metabolite which required further characterization.
 Unfortunately, efforts to chemically synthesize analytical quantities of material were unsuccessful. Separately, in vivo rat bile duct cannulated studies revealed abundant excretion of the glucuronide in bile enabling milligram quantities to be isolated. The material obtained from bile excreta was of sufficient quantity and purity to obtain an NMR spectrum that confidently revealed an acyl glucuronide structure. Material from rat was also sufficient to test in an acyl migration assay. To support future bioanalytical method development and validation needs for clinical studies, significantly larger quantities were required.
Unfortunately, efforts to chemically synthesize analytical quantities of material were unsuccessful. Separately, in vivo rat bile duct cannulated studies revealed abundant excretion of the glucuronide in bile enabling milligram quantities to be isolated. The material obtained from bile excreta was of sufficient quantity and purity to obtain an NMR spectrum that confidently revealed an acyl glucuronide structure. Material from rat was also sufficient to test in an acyl migration assay. To support future bioanalytical method development and validation needs for clinical studies, significantly larger quantities were required.
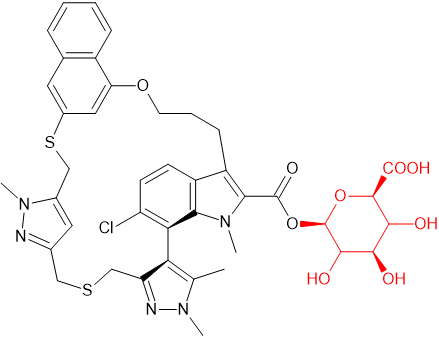
Toward that end, AZD5991 was screened against a panel of microbes at Hypha Discovery which revealed extensive metabolism via phase I and phase II pathways. As observed in pre-clinical studies, a prominent glucuronide was formed by several microbes. The glucuronide produced was demonstrated by LC-MS/MS and NMR to match that observed from human hepatocyte incubations and excreta in rat bile. A 10L scale up of one biotransformation reaction yielded 109.6 mg of the purified acyl glucuronide of AZD5991.
AZD5991 is a potent and selective inhibitor of Mcl-1 which entered clinical development for treatment of haematological malignancies1. Preclinical metabolite profiling of AZD5991 in human hepatocytes revealed the presence of an abundant glucuronide metabolite which required further characterization. Unfortunately, efforts to chemically synthesize analytical quantities of material were unsuccessful. Separately, in vivo rat bile duct cannulated studies revealed abundant excretion of the glucuronide in bile enabling milligram quantities to be isolated. The material obtained from bile excreta was of sufficient quantity and purity to obtain an NMR spectrum that confidently revealed an acyl glucuronide structure. Material from rat was also sufficient to test in an acyl migration assay. To support future bioanalytical method development and validation needs for clinical studies, significantly larger quantities were required. Toward that end, AZD5991 was screened against a panel of microbes at Hypha Discovery which revealed extensive metabolism via phase I and phase II pathways. As observed in pre-clinical studies, a prominent glucuronide was formed by several microbes. The glucuronide produced was demonstrated by LC-MS/MS and NMR to match that observed from human hepatocyte incubations and excreta in rat bile. A 10L scale up of one biotransformation reaction yielded 109.6 mg of the purified acyl glucuronide of AZD5991.