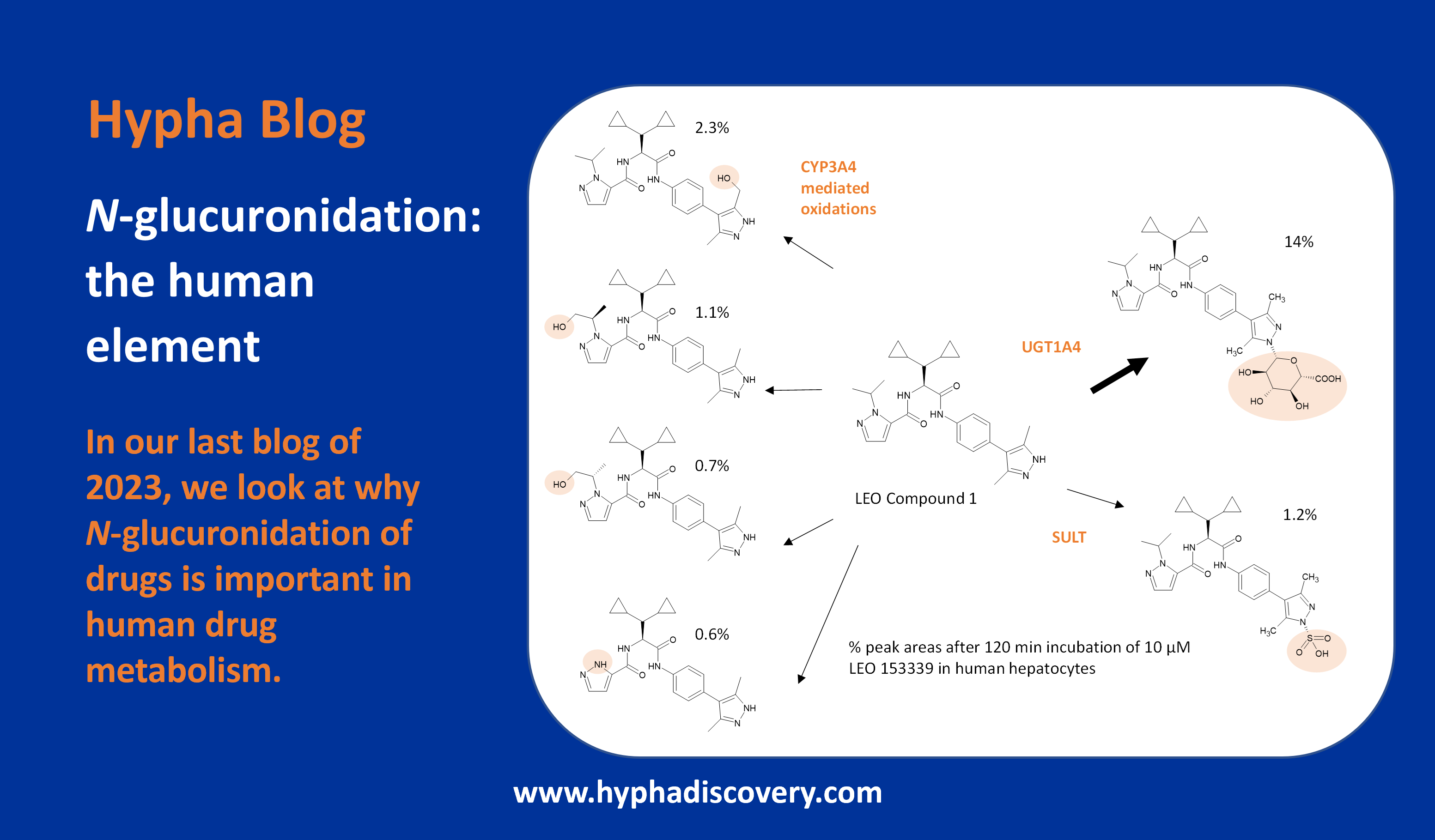
Metabolic pathways and disposition of Tropifexor in humans
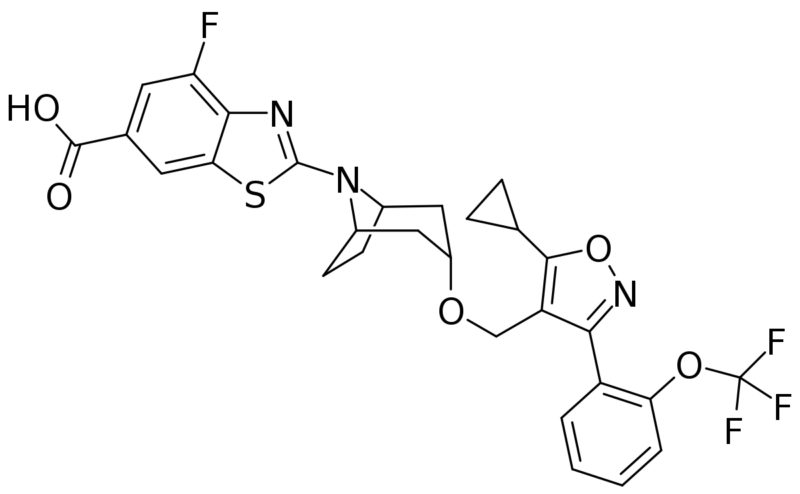
Looking at the structure of tropifexor, a farnesoid X receptor (FXR) agonist for the treatment of nonalcoholic steatohepatitis (NASH), immediately raises thoughts about formation of an acyl glucuronide metabolite.
In fact, tropifexor is predominantly eliminated by metabolism and indeed the acyl glucuronide (M22) was observed during in vitro studies, although not in the human radiolabelled ADME study described in the paper by scientists at Novartis (see reference below).
Investigations with M22 showed that this acyl glucuronide is stable in human plasma with < 5% hydrolyzed back to the parent by 5 hours during an incubation, and in addition a low rate of intramolecular acyl migration under the incubation conditions was evident from mass spec studies. However, one concern with biliary excreted glucuronides is that they can revert to the parent drug through the action of β-glucuronidase secreted by bacteria in the gut.
An ex vivo experiment with M22 showed it was almost entirely converted back to tropifexor after 20 hours of incubation under anaerobic conditions, so providing a plausible explanation as to why this metabolite was not detected in human in vivo experiments. The authors suggest that M22 could have been formed in the liver, excreted in the bile and passed back into the intestinal tract, where it was de-conjugated by β-glucuronidase expressed in microflora, and observed as intact tropifexor in faeces.
Interestingly it turns out that the primary metabolic clearance pathway is dependent on the dose of the drug, with a switch from glucuronidation at higher concentrations to oxidative metabolism dominating at the more clinically relevant lower doses, highlighting the importance of the interplay between different biotransformation mechanisms in the body.
The authors point out that in vivo and in vitro experiments should be carefully designed for optimum understanding of disposition processes during drug development.
Reference
Evaluation of the Absorption, Metabolism, and Excretion of a Single Oral 1 mg Dose of Tropifexor in Healthy Male Subjects and the Concentration Dependency of Tropifexor Metabolism. Wang-Lakshman et al. Drug Metabolism and Disposition May 5, 2021, DMD-AR-2020-000349; DOI: https://doi.org/10.1124/dmd.120.000349