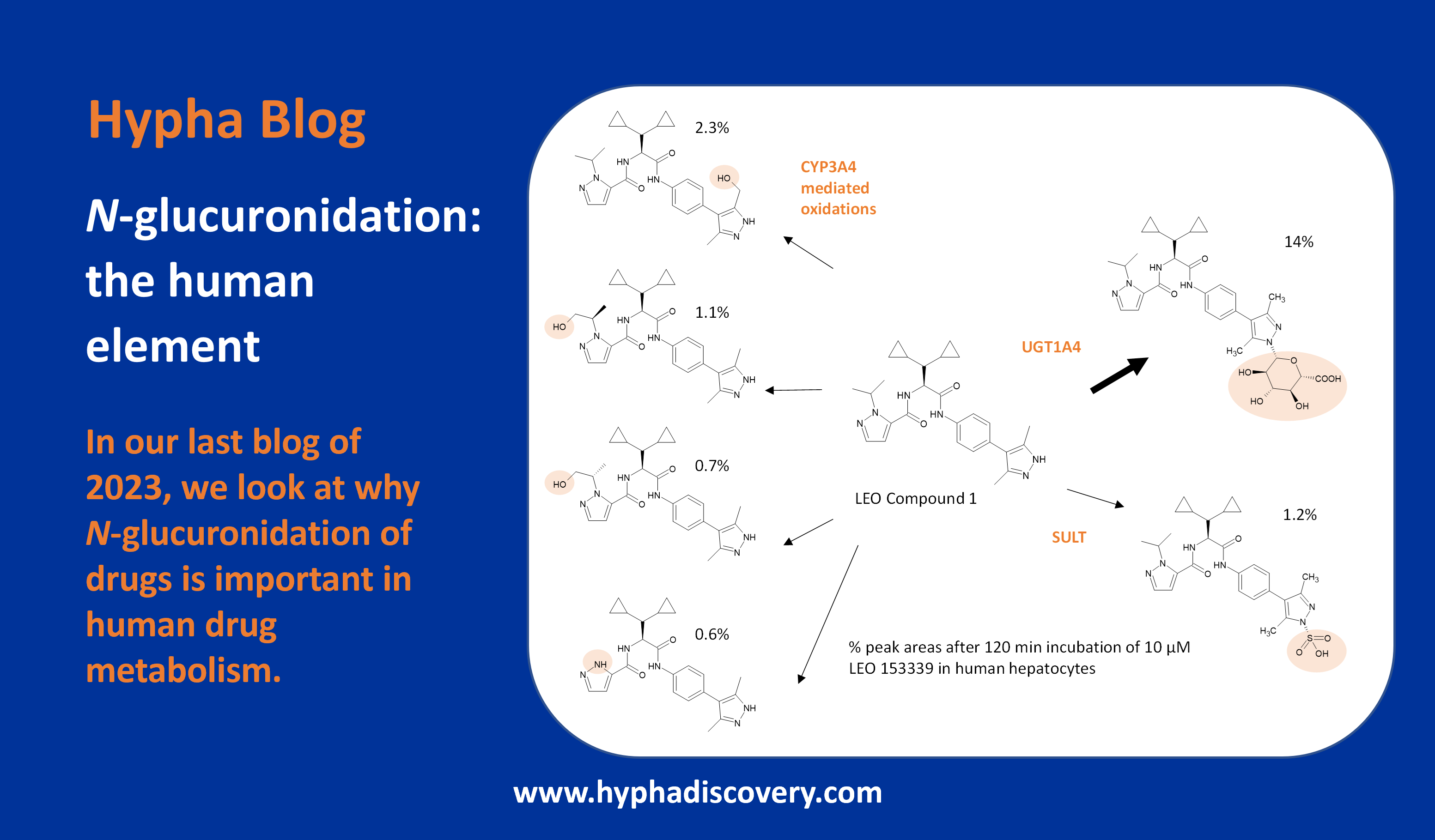
Metabolism of cyclopropyl groups: a double-edged sword
By Julia Shanu-Wilson
Cyclopropyl groups are an appealing substituent to use in drug design because they impart constraint in aliphatic systems while retaining a high fraction of sp3. Furthermore, the ring strain produces shorter, stronger, more polarised C-H bonds that impart some novel properties. The ring system features in numerous FDA-approved small molecule drugs, with many more in preclinical and clinical trials.1 The high C-H bond dissociation energy results in a reduced susceptibility to oxidative metabolism by cytochrome P450 (CYP) enzymes due to the increase in energy needed for the initial hydrogen atom abstraction step.2,3 The classic example of pitavastatin uses a cyclopropyl ring to divert metabolism away from CYP3A4 in favour of clinically insignificant minimal metabolism by CYP2C9, thus reducing potential DDIs.4
Recently scientists at Merck used an iterative exploration of metabolism to pinpoint metabolic hotspots in an IDO1 inhibitor. This resulted in two different cyclopropyl ring systems being proposed to reduce oxidative metabolism, increase the half-life of the drug, and improve potency. 5
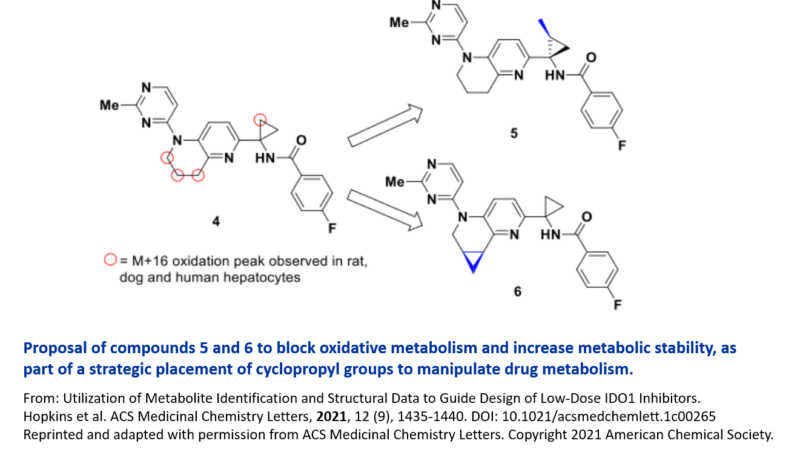
As part of the metabolite identification studies, a surprising oxidation of the cyclopropyl ring was uncovered during the initial optimization. This was observed in rat, dog and human hepatocytes and was a predominant metabolic pathway in rat. To block this oxidation and improve metabolic stability, the cyclopropyl ring was substituted with a methyl group that had the additional benefit of reaching deeper into a lipophilic pocket, boosting potency and LLE.
A significant number of cyclopropyl groups are directly bound to amines. Metabolism of these can result in undesirable biotransformations, such as CYP mediated bioactivation to form GSH conjugates. The well-known example of trovafloxacin, a fluoroquinolone antibiotic associated with hepatotoxicity, involves CYP1A2 mediated oxidation of the cyclopropylamine. This results in the formation of reactive ring-opened intermediates capable of forming covalent adducts to hepatic proteins.6
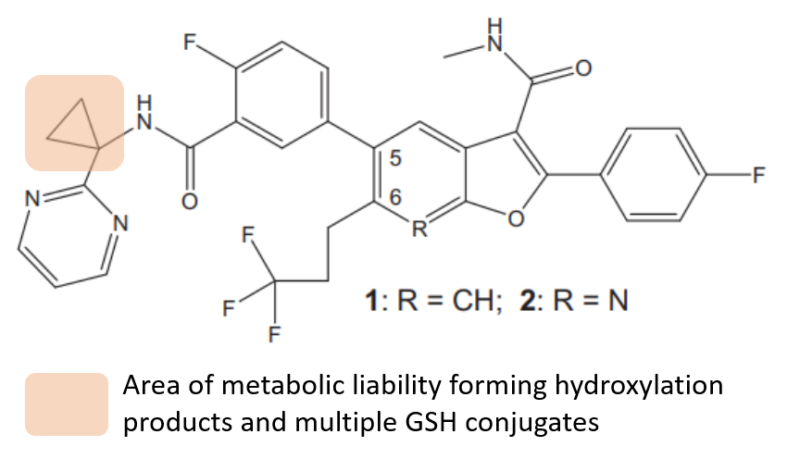 More recently, the mechanism at play in a series of hepatitis C NS5B inhibitors featuring a cyclopropyl motif was described by scientists at BMS.7 They discovered that the cyclopropyl moiety undergoes NADPH-dependent oxidation to form both hydroxylated metabolites and multiple GSH conjugates, indicating the propensity of this ring system to form reactive metabolites. Multiple mechanisms were proposed that explained the GSH conjugates observed, specifically radical formation and ring opening. Medicinal chemists responded by replacing the cyclopropyl ring with a gem-dimethyl group, thereby averting the bioactivation reaction.
More recently, the mechanism at play in a series of hepatitis C NS5B inhibitors featuring a cyclopropyl motif was described by scientists at BMS.7 They discovered that the cyclopropyl moiety undergoes NADPH-dependent oxidation to form both hydroxylated metabolites and multiple GSH conjugates, indicating the propensity of this ring system to form reactive metabolites. Multiple mechanisms were proposed that explained the GSH conjugates observed, specifically radical formation and ring opening. Medicinal chemists responded by replacing the cyclopropyl ring with a gem-dimethyl group, thereby averting the bioactivation reaction.
Roche’s RNA splice modifier risdiplam, approved in August 2020 for the treatment of spinal muscular atrophy, contains a cyclopropyl group. This motif was added to help improve the properties of the original clinical candidate RG7916, which suffered some toxicity liabilities in the clinic. Interestingly, an active N-dealkylated metabolite of RG7916 also caused problems. The metabolite circulated at 9% in plasma vs the parent compound and was 10-fold more potent in vitro, yet was only peripherally available due to being a strong P-gp substrate. Optimisation of the structure of RG7916 involved 3 main changes; removal of the vulnerable methyl group to mitigate against the formation of peripherally restricted active metabolites, introduction of a cyclopropyl group to lower basicity thereby eliminating hERG and phospholipidosis liabilities, and, introduction of a novel imidazopyridazine moiety to eliminate phototoxicity and increase potency.8
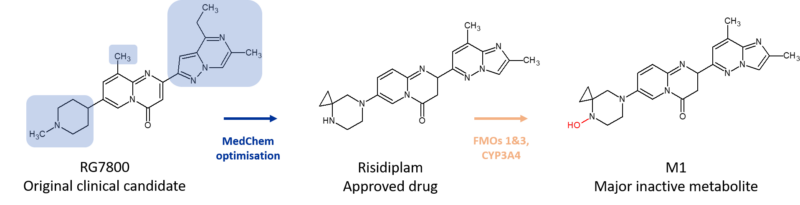
Despite the structural changes, risdiplam is still biotransformed to a major metabolite (M1), which circulates at 14% in plasma but is fortunately inactive. Formation of M1 is largely mediated by FMOs 1 and 3 as well as CYP3A4, avoiding any DDI liabilities. Notably M1 is not seen as a major metabolite in hepatocytes or liver microsomes.
It’s clear that cyclopropyl groups can be used advantageously to diminish oxidative metabolism in some circumstances, but when coupled to amines can lead to potentially reactive intermediates. It will be interesting to see the metabolism stories that emerge during development of the many cyclopropyl containing drugs currently in the clinic.
References
1 Metabolism and Bioactivation: It’s Time to Expect the Unexpected. James P. Driscoll, Corinne M. Sadlowski, Nina R. Shah, and Antonio Feula. Journal of Medicinal Chemistry 2020 63 (12), 6303-6314. DOI: 10.1021/acs.jmedchem.0c00026
2The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. Tanaji T. Talele. Journal of Medicinal Chemistry, 2016, 59 (19), 8712-8756. DOI: 10.1021/acs.jmedchem.6b00472
3Put a ring on it: application of small aliphatic rings in medicinal chemistry. Matthias R. Bauer, Paolo Di Fruscia, Simon C. C. Lucas, Iacovos N. Michaelides, Jennifer E. Nelson, R. Ian Storer and Benjamin C. Whitehurst. RSC Med. Chem., 2021, 12, 448. DOI: 10.1039/d0md00370k
4Pitavastatin: a different pharmacological profile. Alberico L Catapano. Clinical Lipidology, 2021, 7:sup1, 3-9. DOI: 10.2217/clp.12.21
5Utilization of Metabolite Identification and Structural Data to Guide Design of Low-Dose IDO1 Inhibitors. Brett Hopkins, Hongjun Zhang, Indu Bharathan, Derun Li, Qinglin Pu, Hua Zhou, Theodore A. Martinot, Xavier Fradera, Alfred Lammens, Charles A. Lesburg, Ryan D. Cohen, Jeanine Ballard, Ian Knemeyer, Karin Otte, Stella Vincent, J. Richard Miller, Nicolas Solban, Mangeng Cheng, Prasanthi Geda, Nadya Smotrov, Xuelei Song, David Jonathan Bennett, and Yongxin Han. ACS Medicinal Chemistry Letters, 2021, 12 (9), 1435-1440. DOI: 10.1021/acsmedchemlett.1c00265
6In Vitro Metabolism of a Model Cyclopropylamine to Reactive Intermediate: Insights into Trovafloxacin-Induced Hepatotoxicity. Qin Sun, Ran Zhu, Frank W. Foss, and Timothy L. Macdonald. Chemical Research in Toxicology 2008, 21 (3), 711-719. DOI: 10.1021/tx7003085
7Bioactivation of cyclopropyl rings by P450: an observation encountered during the optimisation of a series of hepatitis C virus NS5B inhibitors. Zhuo et al. Xenobiotica, 2018, 48:12, 1215-1226. DOI: 10.1080/00498254.2017.1409915
8 Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA). Ratni et al. Journal of Medicinal Chemistry 2018 61 (15), 6501-6517. DOI: 10.1021/acs.jmedchem.8b00741