Purification of Carisbamate Glucuronides from Urine and Assignment of R and S Epimers by NMR
If clearance mechanisms of the test drug results in sufficient quantities of the major metabolites in biological material such as faeces or urine, purification and subsequent identification of metabolites from such matrices is possible.
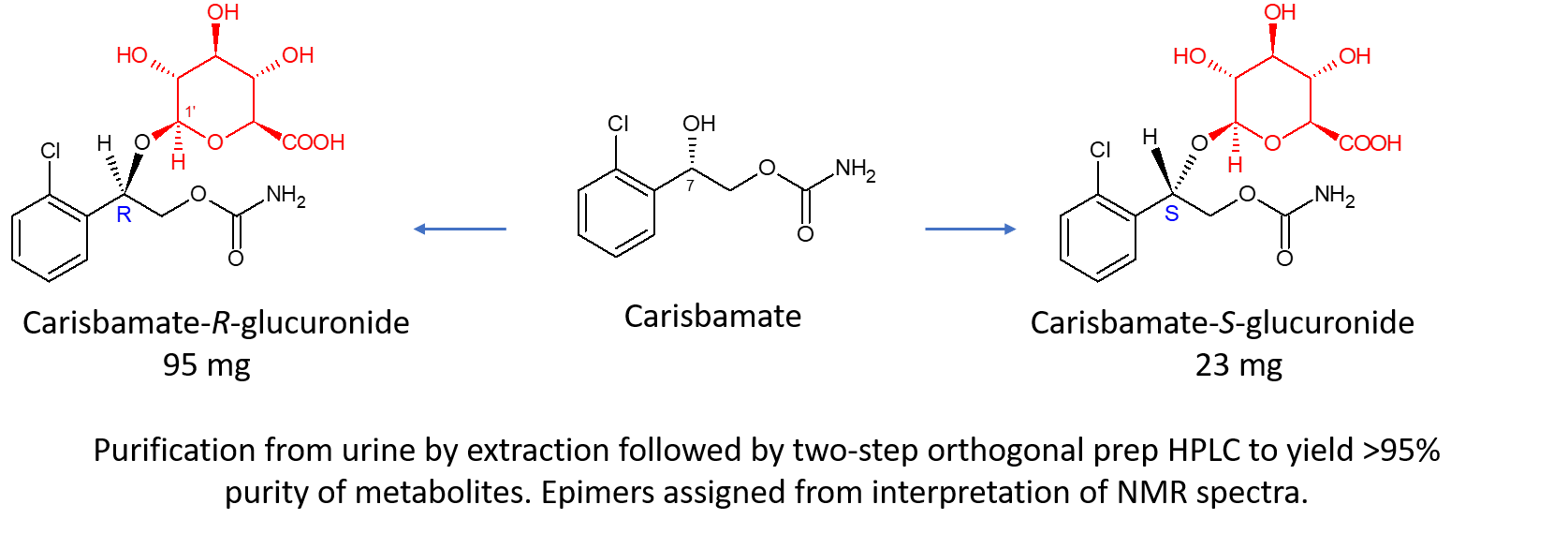
One such project undertaken at Hypha resulted in tens of milligrams of the R- and S-O-glucuronides of carisbamate, a neuromodulator developed by SK Life Science, which were purified to >95% purity from 150 ml of urine using a three-step purification method.
Purification
The initial step involved treating the urine with acetonitrile to precipitate proteins and salts, which were removed by centrifugation. After removal of the acetonitrile by evaporation, the supernatant was purified by preparative reversed phase HPLC, initially by applying it directly to an Xbridge C18 OBD column, which was eluted with a shallow water-acetonitrile gradient. The fractions containing the target glucuronides were concentrated and separated by further reversed phase HPLC on a semi-preparative Xbridge prep phenyl column eluted with a very shallow water methanol gradient. Concentration to dryness of the eluate fractions containing the two target metabolites yielded 95 and 23 mg of the R– and S-glucuronides, respectively. Confirmation of the structures was obtained by NMR spectroscopy, enabling the use of the purified materials as analytical standards for bioanalysis.
Structure elucidation by NMR
COSY, HSQC and HMBC NMR spectra were obtained for both metabolites, with clear HMBC correlations from the 7-methine protons with the glucuronide carbons and from the glucuronide anomeric protons with the 7-methine carbons indicating glucuronidation of the 7-hydroxyl group in both instances. The two metabolites were therefore epimers and there were clear differences in their NMR spectra with respect to the chemical shifts and coupling constants for some protons.
In the NOESY spectra (see expansion in the downloadable pdf of 1H-1H NOESY spectra in DMSO-d6) there was one clear nOe correlation between the 7-methine and glucuronide anomeric protons, and this metabolite was proposed to be R-carisbamate glucuronide. There was no corresponding correlation evident in the NOESY spectrum of the second metabolite, which was therefore proposed to be S-carisbamate glucuronide.
Related Resources
In recent years, FDA guidance has advised initiating human metabolite profiling earlier in drug development, emphasizing the importance of metabolite identification and quantification to evaluate a drug metabolite’s safety and pharmacological activity. Praliciguat (IW-1973) is a soluble guanylate cyclase (sGC) stimulator in Phase 2 clinical trials for diabetic nephropathy and heart failure with preserved ejection fraction (HFpEF). During studies on metabolism of praliciguat in preclinical species and in human hepatocytes, a prominent direct O-glucuronide metabolite was detected.
Access to multiple metabolites needed to support clinical development is not always straightforward, and can sometimes mean that more than one technique needs to be applied to fulfil requirements. In one such project, a US pharma client required > 200 mg of three metabolites of a drug; an N-glucuronide (M1), an indirect O-glucuronide (M2b) and a hydroxylated metabolite (M8b). As part of this project, multiple components of Hypha’s one-stop metabolite shop were employed, including chemical synthesis, microbial biotransformation as well as purification and structure elucidation by NMR.
In Chapter 4 of the book on “Identification and quantification of drugs, metabolites, drug metabolizing enzymes and transporters”, Hypha authors summarise the different methods employed for producing metabolites of drugs, illustrated with representative examples from the literature and work undertaken at Hypha. The chapter also includes a discussion and examples of the use of NMR spectroscopy for structure elucidation of metabolites.


