Paper Pick – The application of mechanistic absorption, distribution, metabolism and excretion studies and physiologically-based pharmacokinetic modeling in the discovery of the next-generation oral selective estrogen receptor degrader camizestrant to achieve an acceptable human pharmacokinetic profile
In this paper by Gangl and colleagues at AstraZeneca, the integration of multiple pieces of in vitro data, in vivo PK and modelling to generate a satisfactory PK prediction for human dosing of camizestrant is nicely demonstrated, highlighting the importance of having a thorough understanding of clearance rates and mechanisms across species to predict in vivo outcomes.
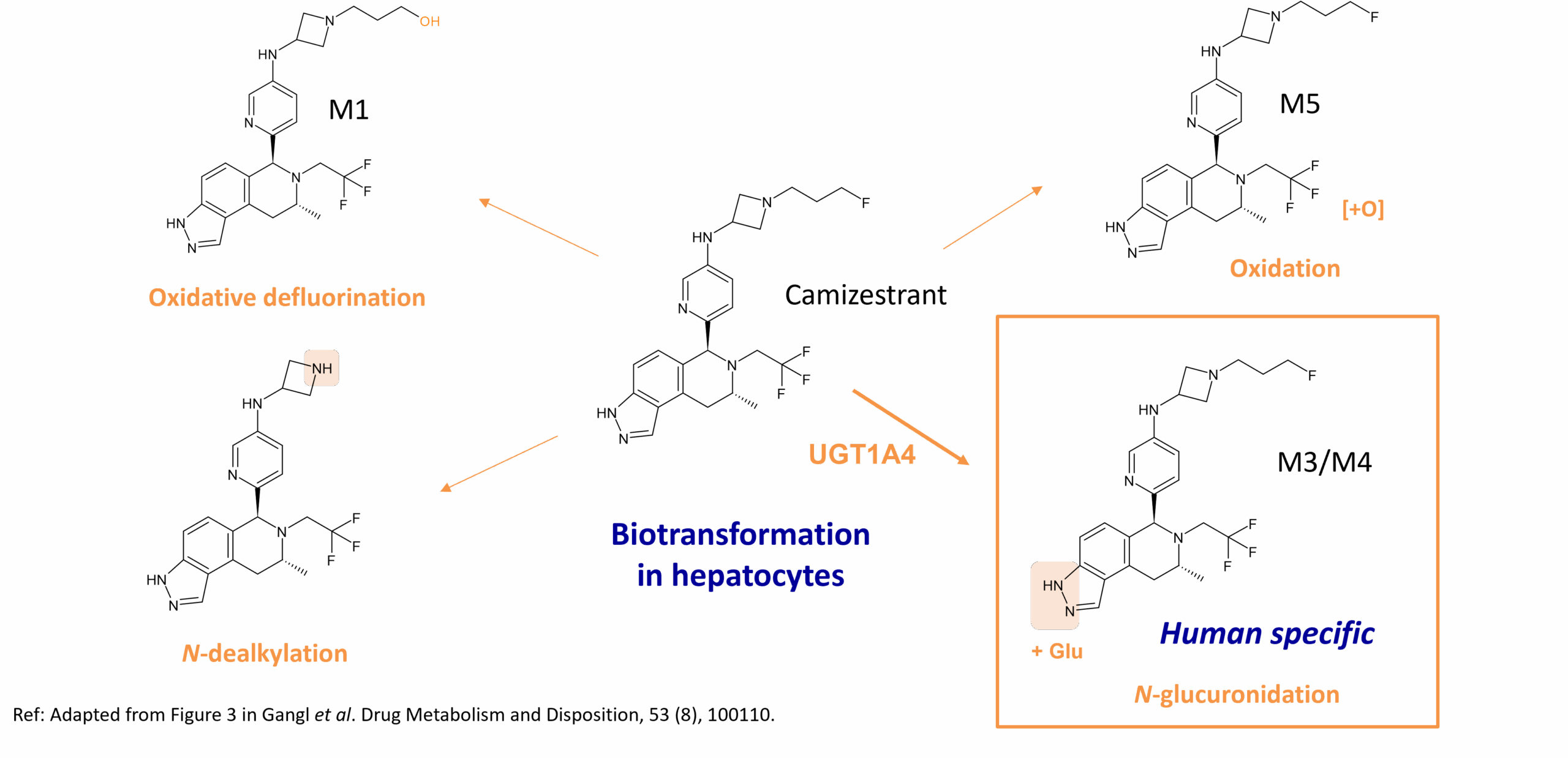
Camizestrant (AZD9833) is a potent SERD and full ERα antagonist and is currently in phase 3 clinical trials for the treatment of ER+ breast cancer. Clearance of the drug differs between rat, dog and human with human clearance showing less dose dependence than in dogs. Moreover, routes of biotransformation differ in human hepatocytes with UGT1A4 driving human-specific N-glucuronidation (M3/M4), in addition to oxidative routes observed in other animal species. Oxidative pathways include oxidation (M5), oxidative defluorination (M1) and N-dealkylation (M2) which in humans is mediated mainly by CYP3A4. In fact, a point is made that the human-specific N-glucuronidation of camizestrant is consistent with other drugs metabolized by UGT1A4 and UGT2B10 where rates of N-glucuronidation can be much lower in preclinical species compared to humans.
Paper
Gangl ET, Markandu R, Sharma P, et al. The application of mechanistic absorption, distribution, metabolism and excretion studies and physiologically-based pharmacokinetic modeling in the discovery of the next-generation oral selective estrogen receptor degrader camizestrant to achieve an acceptable human pharmacokinetic profile. Drug Metab Dispos. 2025;53(8):100110. doi:10.1016/j.dmd.2025.100110