Metabolite Synthesis
Synthesis of glucuronides
Glucuronide synthesis
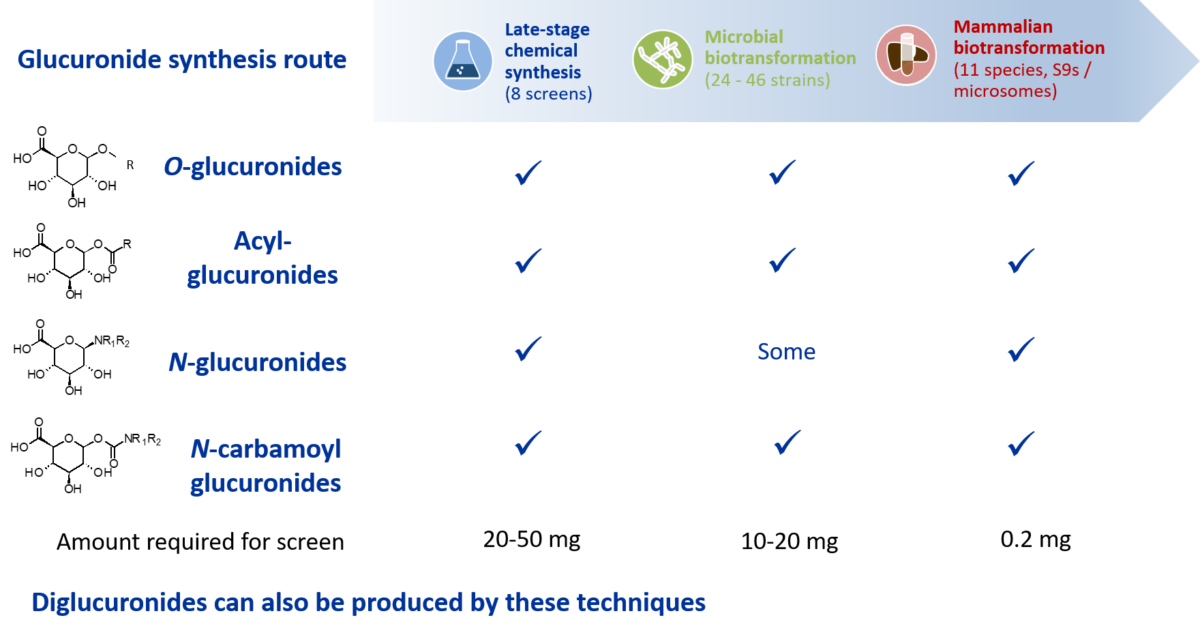
Hypha solves the challenges in glucuronide synthesis using multiple tools from our one-stop metabolite shop. These include microbial biotransformation, mammalian S9 / microsome preparations and our proprietary late-stage chemical synthesis screens, which are scalable to provide gram amounts of O-, acyl, N– and N-carbamoyl glucuronides, as well as other conjugates such as glycosides and sulfates. Diglucuronides and secondary glucuronides can also be produced by these techniques. Microbial biotransformation is particularly suited to producing glucuronides that arise from sequential reactions, such as hydroxylation followed by O-glucuronidation.
We apply the one-stop shop techniques in parallel to determine the most efficient and cost-effective conversion to the glucuronide(s) of interest before proceeding to scale-up synthesis. This can determine the optimum system for production of a single glucuronide, or where multiple glucuronides are required, the best system for scale-up of each conjugate. For example, in one client project, screening revealed that 3 of 4 required glucuronides could be made using late-stage chemical synthesis methods, with the other only provided through a liver S9 biotransformation route.
What We Do
Purification of glucuronides
We specialise in purifying glucuronides (and other metabolites) from various biological matrices and complex mixtures. This is possible even where small amounts of metabolite are present, such as in one client project where we isolated 0.4 mg of a glucuronide from 24L of urine. The pure material was subsequently used to determine the structure by 2D NMR spectroscopy.
Our scientists are experts in NMR data interpretation. We use data acquired on a 700 MHz machine equipped with a 1.7 mm cryoprobe. Data is tabulated to publication standard suitable for regulatory submission.
Why is glucuronidation an important route of drug metabolism?
Glucuronidation is the predominant phase 2 metabolic route for a variety of drugs and agrochemicals catalysed by UDP-glucuronosyltransferases (UGTs) into hydrophilic conjugates that are eliminated from the cell by efflux transporters. UGTs are a family of membrane-bound proteins located in the endoplasmic reticulum in the cells of various tissues, with UGT 1A and UGT 2B subfamilies responsible for most drug metabolism.
Although many UGTs are expressed in human liver, some are also expressed extrahepatically and some are only expressed in certain organs (Ge et al., 2016). UGTs catalyse the transfer of glucuronic acid to a variety of O-, N-, C– and S– containing parent substrates and have become increasingly of interest in the elimination of drugs.
Although generally pharmacologically inactive, some acyl glucuronides of carboxylic acid containing drugs can form acyl migration isomers, which result in transacylation and glycation reactions with proteins causing idiosyncratic adverse drug reactions.
Several classes of glucuronides have been shown to interact with CYPs, in particular CYP2C8 due to its distinctive active site (Ma et al., 2017).
Some glucuronides are subject to enterohepatic recycling, such as the O-glucuronide of epacadostat made by one of Hypha’s microbial strains (Boer et al., 2016), where hydrolysis of the glucuronide by gut bacteria can occur. This is followed by the absorption of the drug back into systemic circulation, thereby leading to a prolonged elimination half-life and altered pharmacokinetics and pharmacodynamics.
Glucuronide synthesis - client case studies
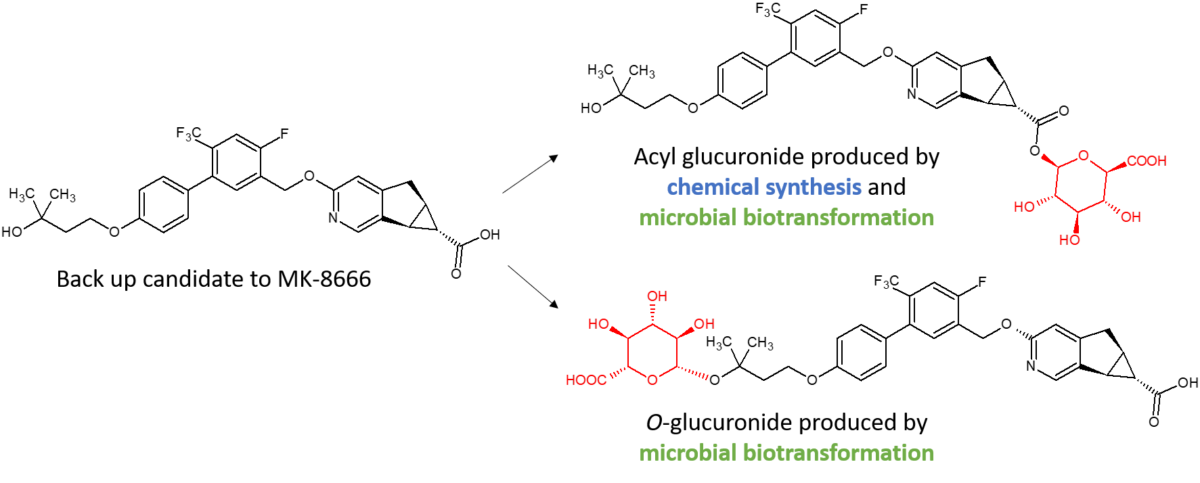
Synthesis of an O-glucuronide of MK-8666 by microbial biotransformation
Clearance of a GPR40 partial agonist backup candidate to MK-8666 by glucuronidation had the potential for conjugation to occur either at the alcohol functionality (ether glucuronide) or at the carboxylic acid (acyl glucuronide). Synthesis of both glucuronides was needed for stability testing. Although the chemical synthesis of the acyl glucuronide was facile, the tertiary alcohol was refractory to generation of the O-glucuronide, presumably due to steric hindrance. Microbial biotransformation was successful in generating both glucuronides (Salter et al., 2018). Synthesis of the O-glucuronide through a small 50 ml scale microbial biotransformation yielded 0.46 mg of pure material, the structure of which was confirmed by NMR spectroscopy. The O-glucuronide synthesised was shown to have identical chromatographic retention time and molecular ion to a reference peak produced by incubation of the parent drug with recombinant human UGT 1A4.
Synthesis of an acyl-glucuronide of the macrocycle AZD5991 by microbial biotransformation
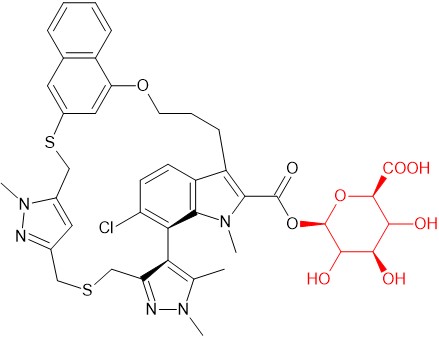
AZD5991 is a potent and selective inhibitor of Mcl-1 which entered clinical development for treatment of haematological malignancies1. Preclinical metabolite profiling of AZD5991 in human hepatocytes revealed the presence of an abundant glucuronide metabolite which required further characterization.
In order to obtain scalable quantities of the glucuronide, AZD5991 was screened against a panel of microbes at Hypha, revealing extensive metabolism via phase I and phase II pathways. As observed in pre-clinical studies, a prominent glucuronide was formed by several microbes. The glucuronide produced was demonstrated by LC-MS/MS and NMR to match that observed from human hepatocyte incubations and excreta in rat bile. A 10L scale up of one biotransformation reaction yielded 109.6 mg of the purified acyl glucuronide of AZD5991.
Download a poster on this work presented at Drug Discovery Chemistry in 2023.
Why access glucuronides?
For pharmacological testing
For definitive structure elucidation
As authentic standards for bioanalysis & quantification
For stability / reactivity testing
For a role in any DDIs arising from transporter-mediated effects
Involvement in enterohepatic recirculation
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
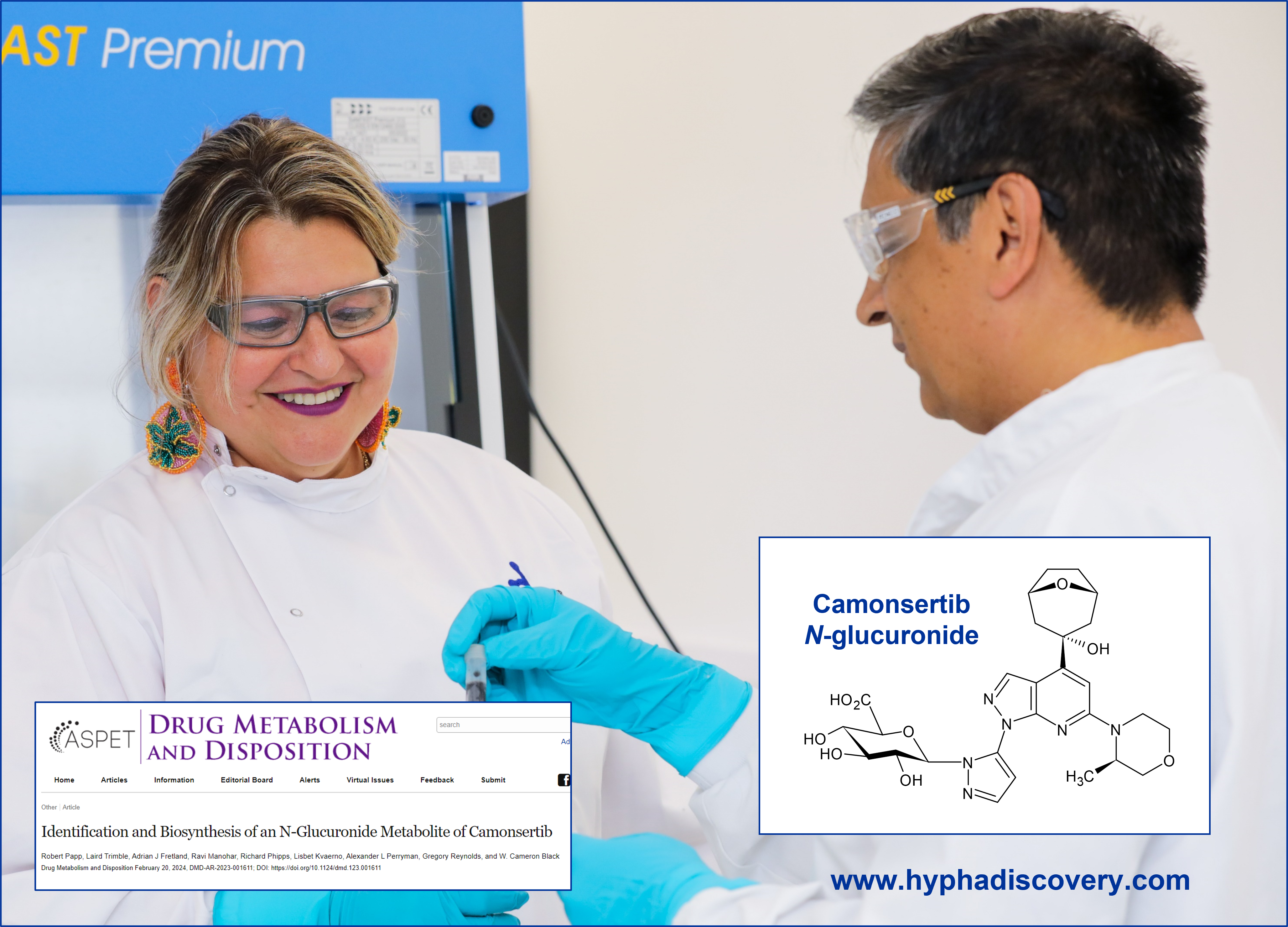
This joint paper published by scientists at Repare Therapeutics, BioAgilytix and Hypha Discovery is the first report of a N-glucuronide metabolite of camonsertib formed in human hepatocyte incubations. The structure of the metabolite was elucidated by NMR after scale-up using microbial biotransformation to produce the N-glucuronide.
In recent years, FDA guidance has advised initiating human metabolite profiling earlier in drug development, emphasizing the importance of metabolite identification and quantification to evaluate a drug metabolite’s safety and pharmacological activity. Praliciguat (IW-1973) is a soluble guanylate cyclase (sGC) stimulator in Phase 2 clinical trials for diabetic nephropathy and heart failure with preserved ejection fraction (HFpEF). During studies on metabolism of praliciguat in preclinical species and in human hepatocytes, a prominent direct O-glucuronide metabolite was detected.
The FDA’s 2020 MIST guidance states that phase 2 conjugates are generally pharmacologically inactive, however where a potentially toxic conjugate, such as an acyl glucuronide is formed, additional safety assessments may be needed. Idiosyncratic drug toxicity of carboxylic acid-containing drugs can be caused by the formation of reactive acyl glucuronides, which have the ability to directly acylate proteins and undergo intramolecular rearrangement producing reactive aldehydes leading to protein glycation.
Find out about synthesis of other metabolite types

Hypha Discovery did a fantastic job synthesizing N- and O- glucuronides of our clinical stage drug substance. The project updates were detailed, our questions were answered in a timely manner, and the overall timeline was maintained. Hypha was highly recommended to us and I would not hesitate to recommend them to a colleague.
Joshua Day Ph.D, Director of Chemistry
Crestone, Inc., Colorado, USA
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk