Metabolite Synthesis
Synthesis of Non-CYP Phase 1 Metabolites
Non-CYP Metabolite Synthesis
Non-CYP metabolite synthesis has become an important element in the ability to evaluate the impact of formation of alternative phase 1 metabolites. A consequence of the development of drugs that are less susceptible to some mechanisms of CYP metabolism, has been the increase in drugs that undergo metabolism via alternative non-CYP routes. It is thus important to be able to access metabolites resulting from these pathways to inform the design and optimisation of such drugs.
Non-CYP phase 1 mechanisms involve the modification of drugs by enzymes such as monoamine oxidases, flavin-containing monooxygenases (FMOs), xanthine oxidases, carboxylesterases, carbonyl reductase, N-acetyltransferases and alcohol / aldehyde dehydrogenases. Micro-organisms in Hypha’s biotransformation panel are able to undertake many non-CYP phase 1 reactions, offering a viable solution to producing metabolites formed by these mechanisms.
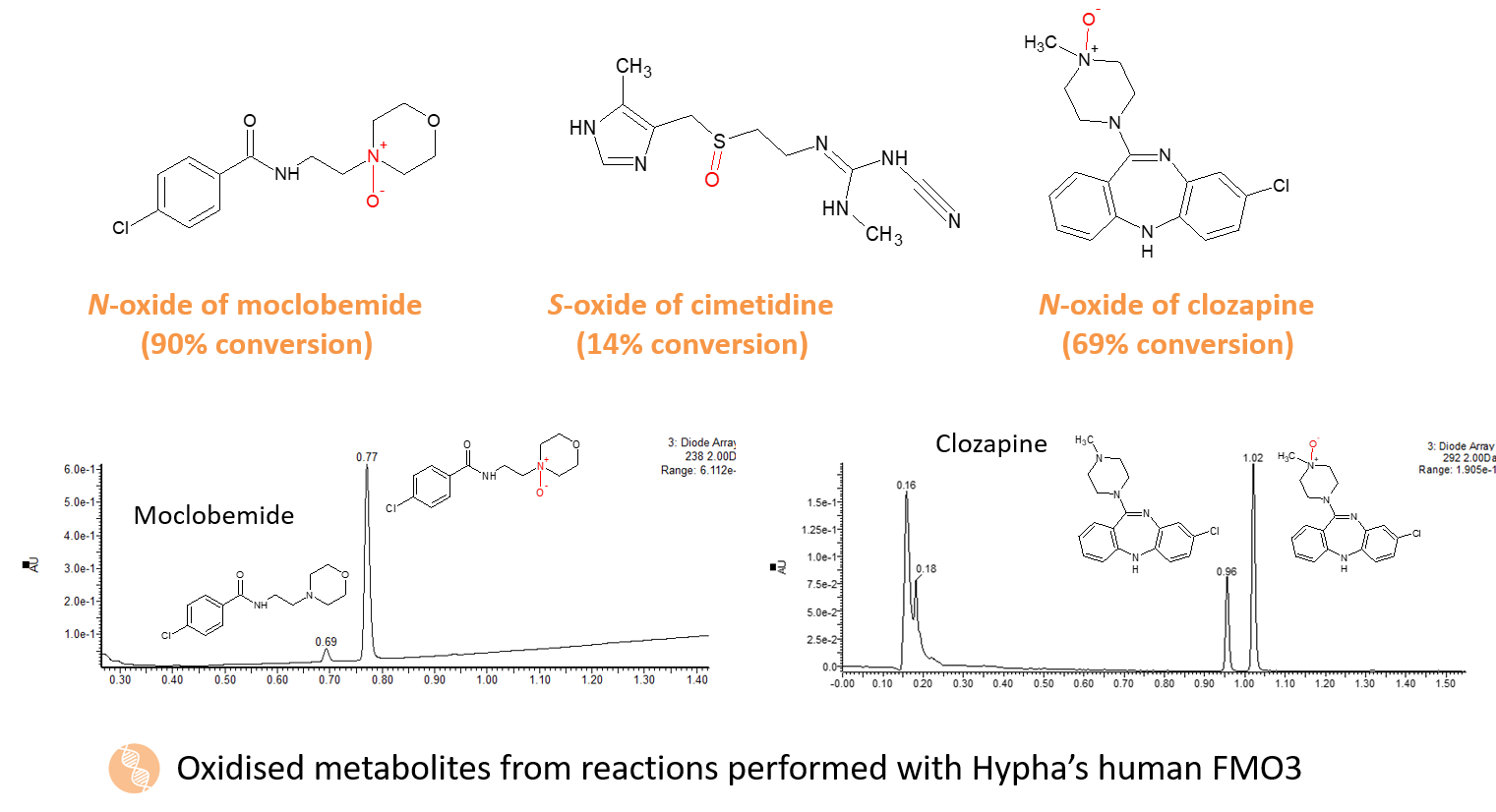
As well as the microbes, we also have available recombinant human aldehyde oxidase and all the human FMO isoforms for synthesis of metabolites formed by these enzymes.
Late-stage chemical methods are also effective for non-CYP metabolite synthesis where oxidation of heteroatoms occurs to form N-oxides and sulfoxides.
What We Do
Recombinant flavin-containing monooxygenases (FMOs)
FMOs play an important and potentially underestimated role in drug metabolism. Since FMOs are not readily induced or inhibited, drugs which are predominantly metabolised by these enzymes may offer clinical advantages (Phillips and Shepherd, 2017). They are a family of NADPH-dependent enzymes that catalyse the oxidation of nucleophilic nitrogen-, sulfur-, phosphorous-, and other heteroatom-containing compounds. N-oxide and S-oxide formation represent the major FMO-mediated reactions. The various FMOs exhibit differential tissue distribution and have different substrate specificity. FMO3 and FMO5 are expressed in the liver and FMO1 and FMO2 are mainly expressed in the kidney and lung respectively.
Metabolites derived from any FMO subtype can now be produced as a service offering at Hypha. Recombinant enzymes of all five forms of human FMOs have been cloned into E.coli to provide a mechanism to access scalable quantities of FMO-derived metabolites.
FMO3, the isoform associated with the bulk of hepatic FMO-mediated metabolism, is also available as part of Hypha’s PolyCYPs+ kit together with 18 PolyCYP isoforms and human AOX1 for accessing phase I oxidised metabolites. FMO3 catalyses N-oxygenation of primary, secondary and tertiary amines, as well as S-oxygenation of nucleophilic sulfur-containing compounds.
Oxidations of azaheterocycles have been reported to contribute to high metabolic clearance and interspecies differences, resulting in the termination of several drug discovery programs in the past. It is therefore important to be aware of any AO-mediated metabolism early on, but not to automatically avoid AO structural flags, since many of them are versatile and valuable building blocks. (Manevski et al., 2019). Hypha have cloned AOX1 into E.coli and demonstrated catalytic activity against several aldehyde oxidase drug substrates. Metabolites derived from human aldehyde oxidase can now be produced as a service offering at Hypha. AOX1 is also included in Hypha’s PolyCYPs+ kit for accessing phase 1 oxidised metabolites.
References
Case Study
Provision of multi grams of an N-oxide metabolite by microbial fermentation
Two major human metabolites of a drug had previously been identified, one derived from metabolism by CYP3A4 and the other largely derived from the action of an FMO. The resulting bis-N-oxide was difficult-to synthesise so microbial biotransformation was used to screen for production of this metabolite. One fungal strain was identified that produced both the correct human-relevant active mono-N-oxide and bis-N-oxide in parallel, where multiple isomers were possible. Altering the relative levels of these two metabolites was achieved by changing the post-dosage harvesting time. Multiple rounds of fermentation and purification resulted in the production of tens of grams of the bis-N-oxide target metabolite, the identity of which was confirmed by NMR.
As well as the microbes, we also have available recombinant human aldehyde oxidase and all the human FMO isoforms for production of metabolites formed by these enzymes.
Find out about synthesis of other metabolite types
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
Hypha’s PolyCYPs kits are in routine use by pharma and agchem companies for producing human and other mammalian metabolites. One application involves use of PolyCYPs for creating radiolabelled metabolites for direct comparison with the radio profiles from mass balance and distribution study samples, necessary for regulatory filing. PolyCYPs provides a clean route for scalable access to more of the CYP-derived metabolites observed in these matrices, for definitive MetID and any tox studies deemed necessary. This is especially useful where low concentrations or unstable metabolites in the mass balance sample make structural identification difficult.
Formation of N-oxide metabolites is one of the major pathways for metabolism of tertiary nitrogen-containing drugs. Some N-oxide metabolites have similar or greater pharmacological activity to the parent drug and thus require exposure assessment. They can also be unstable and can revert to the parent drug.1 Conversion of an N-oxide metabolite back to the parent in vivo is a well- known phenomenon which may result in an altered tissue distribution of the metabolite and parent drug, such as that proposed for tamoxifen,2 or cause adverse reactions as reported for soratenib.3 In the lab, it is possible to reduce the potential for conversion of N-oxide metabolites to the parent through careful sample handling, as described for the clinically-significant metabolite loxapine N-oxide.4
There has been a notable increase in metabolism of new drug candidates through non-CYP phase I pathways such as those mediated via aldehyde oxidase (AO).1 Further, mixed AO/P450 substrates may be subject to metabolic shunting, an important consideration during toxicology and DDI assessment of these drugs.2 Access to metabolites may thus be important to consider for drugs with mixed metabolism.

“The project initially involved purification of milligram amounts of an AO metabolite from litres of human urine. It was subsequently found that the metabolite could be produced by Hypha’s human recombinant AOX1 and by two microbes. One of these microbes could convert the parent drug to the metabolite at a 67% conversion. Following a successful dose escalation experiment, over 800 mgs of the metabolite was purified from a 2L microbial biotransformation.”
Director Clinical Director
US Pharma Company
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk