Regio Selective Formation of N-oxide Metabolites
Phase 1 metabolism of tertiary nitrogen-containing drugs
Formation of N-oxide metabolites is one of the major pathways for metabolism of tertiary nitrogen-containing drugs. Some N-oxide metabolites have similar or greater pharmacological activity to the parent drug and thus require exposure assessment. They can also be unstable and can revert to the parent drug.1 Conversion of an N-oxide metabolite back to the parent in vivo is a well- known phenomenon which may result in an altered tissue distribution of the metabolite and parent drug, such as that proposed for tamoxifen,2 or cause adverse reactions as reported for soratenib.3 In the lab, it is possible to reduce the potential for conversion of N-oxide metabolites to the parent through careful sample handling, as described for the clinically-significant metabolite loxapine N-oxide.4
Active N-oxides
Some N-oxide metabolites have similar or greater pharmacological activity to the parent drug and thus require exposure assessment
Unstable N-oxides
Some N-oxide metabolites are unstable and can revert to the parent drug
Trihexyphenidyl (THP) is an anti-cholinergic agent used to treat Parkinson’s disease and is also subject to drug abuse.5 Major human metabolites of THP constitute hydroxycyclohexyl derivatives and an N-oxide, none of which have been structurally characterized in the literature. Through microbial biotransformation, we were able to prepare and isolate the N-oxide metabolite of THP together with two metabolites monohydroxylated in the cyclohexyl ring. Although the N-oxide of THP can be readily accessed synthetically, where there is potential for N-oxidation at multiple positions, a more selective approach is required.
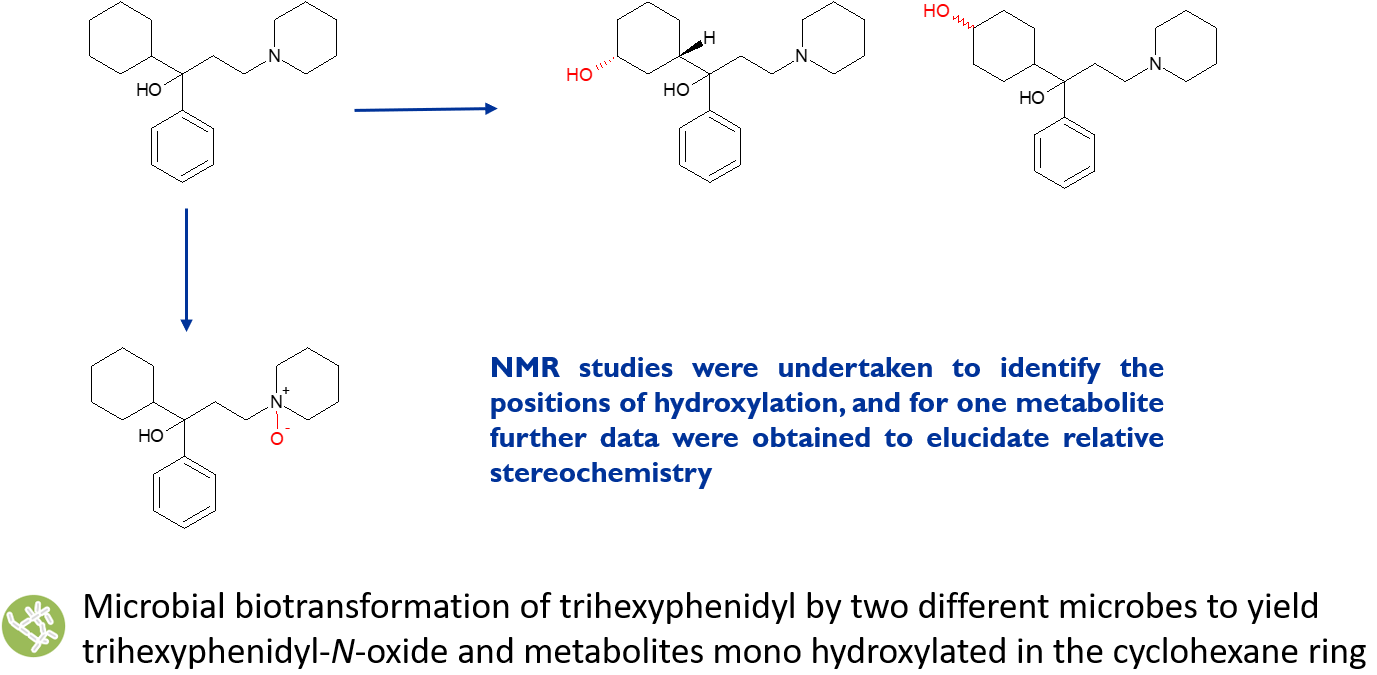
N-oxides are formed through the action of cytochrome P450 and/or flavin-containing monooxygenase (FMO) enzymes. Microbial isoforms of these enzymes can selectively form the homologous human drug metabolites in quantities that allow full structural elucidation and further biological testing.
For a client project, Hypha was able to produce multi-gram amounts of the correct bis-N-oxide, through microbial biotransformation, where multiple isomers were possible. In parallel the correct human-relevant active mono-N-oxide was also produced, which in humans had been shown to be formed primarily via a FMO.
References
1. Majunder, 2013. Regulated Bioassay of N-oxide Metabolites Using LC-MS, in Handbook of LC-MS Bioanalysis: Best practices, Experimental Protocols, and Regulations.
2. Gjerde et al., 2012. Breast Cancer Res. Treat. 134(2), 693-700.
3. Gillani et al., 2015. Chem. Res. Toxicol. 28(1), 92-102.
4. Meng et al., 2017. J. Chromatography B 1046, 87-97
Related Resources
There has been a notable increase in metabolism of new drug candidates through non-CYP phase I pathways such as those mediated via aldehyde oxidase (AO).1 Further, mixed AO/P450 substrates may be subject to metabolic shunting, an important consideration during toxicology and DDI assessment of these drugs.2 Access to metabolites may thus be important to consider for drugs with mixed metabolism.
In Chapter 4 of the book on “Identification and quantification of drugs, metabolites, drug metabolizing enzymes and transporters”, Hypha authors summarise the different methods employed for producing metabolites of drugs, illustrated with representative examples from the literature and work undertaken at Hypha. The chapter also includes a discussion and examples of the use of NMR spectroscopy for structure elucidation of metabolites.
Metabolite identification is an integral part of both preclinical and clinical drug discovery and development. Synthesis of drug metabolites is often required to support definitive identification, preclinical safety studies and clinical trials.


