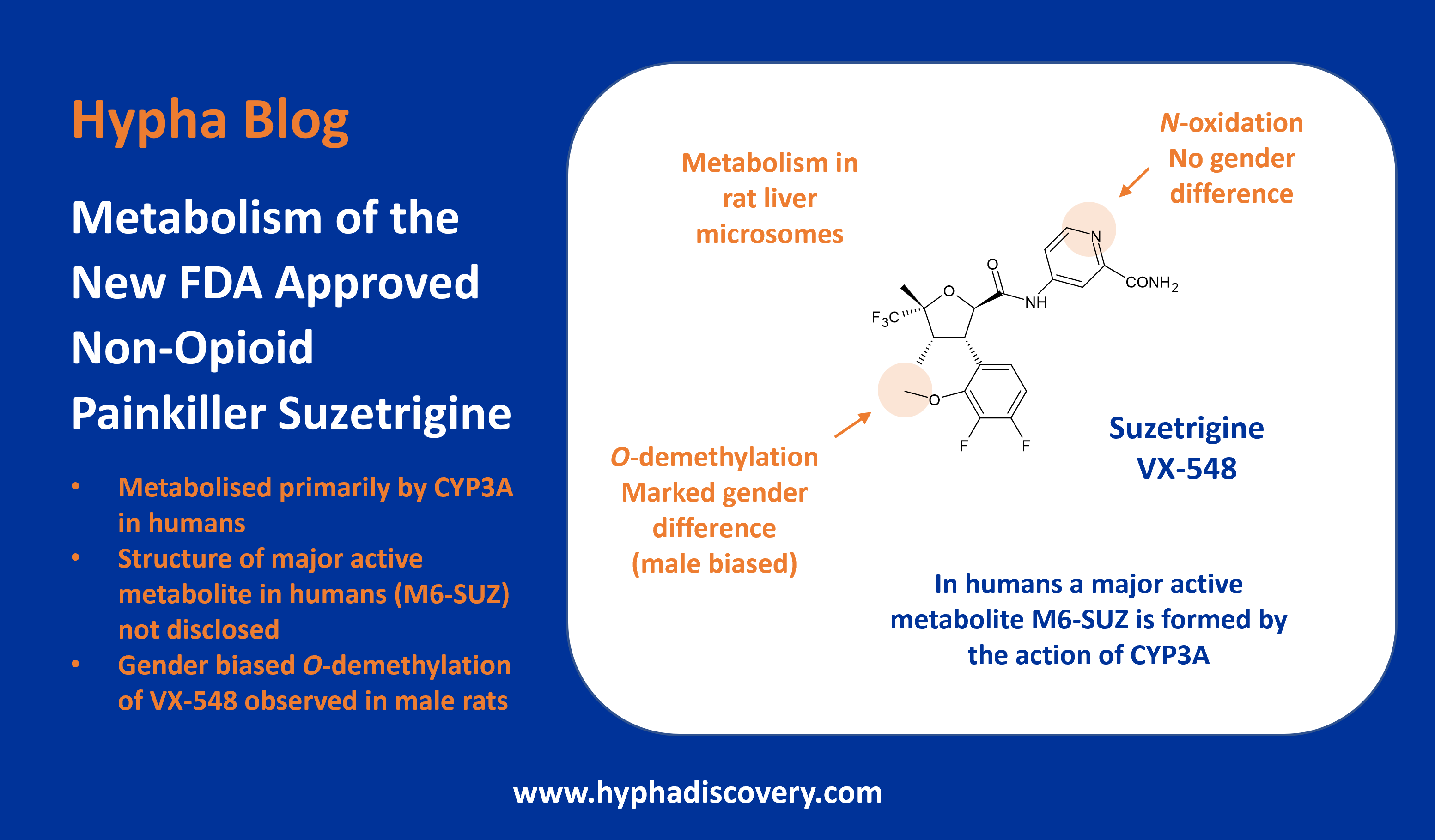
Discovery of unprecedented human stercobilin conjugates of inavolisib
Our highlighted paper this month is one in which Hypha worked with scientists at Genentech to generate and elucidate the structures of novel metabolites of inavolisib observed in human feces. M18 and M19 are conjugated with part structures of stercobilin, a microbial metabolite of bilirubin found in the gut which imparts the brown color to feces. To our knowledge this type of drug conjugate has not been reported before now.
In May 2024 inavolisib (GDC-077) gained breakthrough therapy designation as a result of positive results from a phase 3 study, when dosed in combination with palbociclib and fulvestrant in patients with hormone receptor–positive, HER2-negative locally advanced or metastatic breast cancer harboring a PIK3CA mutation. PIK3CA is the gene encoding the catalytic subunit of the alpha isoform of phosphatidylinositol 3-kinase (PI3Kα).
Metabolism of inavolisib
Inavolisib has high potency and specificity for inhibition of the mutated PI3Kα isoform, where it induces selective degradation of mutated PI3Kα [1]. A radiolabeled mass balance study of the absorption, metabolism and excretion of [14C]inavolisib, presented in a poster at an ISSX meeting in 2023, determined that the parent drug comprises the major circulating component. Curiously, in feces, although the main metabolic pathway in humans was via hydrolysis of the amide moiety to form M8 (Figure 1), several unusual conjugates were also observed in feces which possessed a mass change of parent +302 or +304 Da, with or without amide hydrolysis [2]. Together the 9 metabolites observed with these mass changes comprised nearly 25% of the dose in feces.
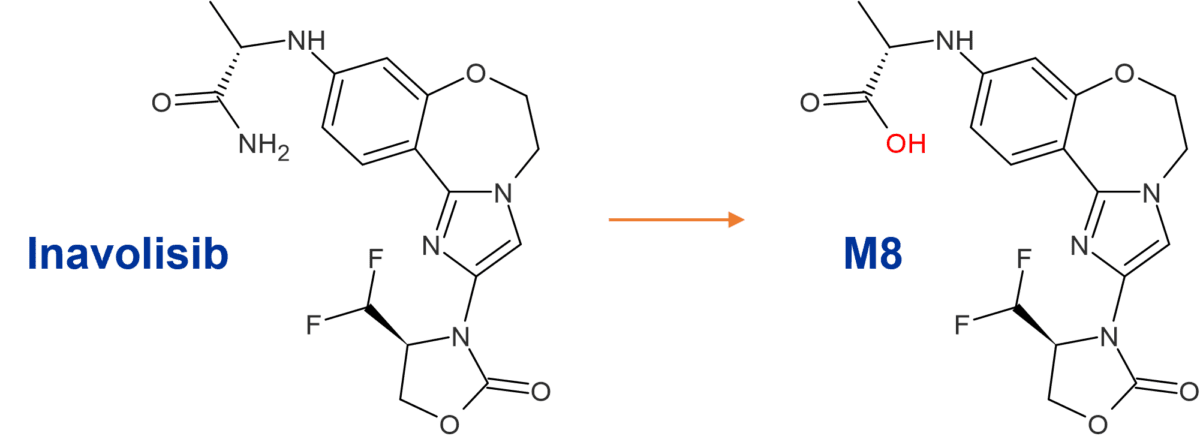
Figure 1: Amide hydrolysis metabolite of inavolisib
Unusual conjugates in feces
An investigation was undertaken to understand the structures of these unusual metabolites, the results of which are reported in this highlighted paper [3]. Interpretation of NMR spectroscopy data obtained on the two non-hydrolyzed +304 Da metabolites M18 and M19 (together comprising 4.2% of dose in feces and 1.3% of dose in urine), revealed novel structures arising from conjugation of the imidazole ring of inavolisib with one half of a stercobilin molecule. M18 and M19 differ only in the positions of the methyl and ethyl groups on the pyrrolidin-2-one moieties. (Figure 2).
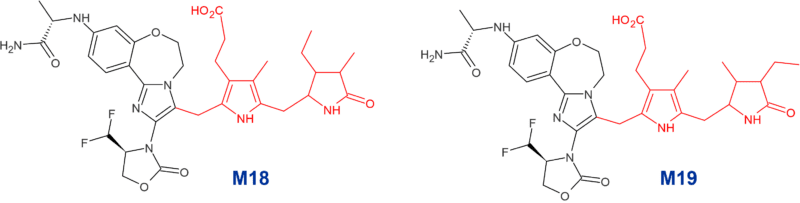
Figure 2: Part-stercobilin conjugated metabolites of inavolisib
Formation of stercobilin in the gut
Stercobilin is a breakdown product of heme and is the component that imparts a brown color to feces. It forms from breakdown of bilirubin excreted into the bile from the liver as a diglucuronide, which is then deconjugated and reduced by gut bacterial enzymes to urobilinogen and stercobilinogen. Stercobilinogen is subsequently oxidised to stercobilin (Figure 3). The first bacterial enzyme involved in the reduction is bilirubin reductase. Interestingly this enzyme is nearly universally present in healthy adults, while the prevalence of the gene is much lower in patients with inflammatory bowel disease and in infants [4]. In another paper, elevated levels of stercobilin have been implicated in pro-inflammatory disease processes such as diabetes in mouse models [5]. The potential impact of varying levels of microbial metabolites of bilirubin raises interesting questions about whether the status of an individual’s gut microbiome may impact on the levels of part-stercobilin conjugates of inavolisib in feces.
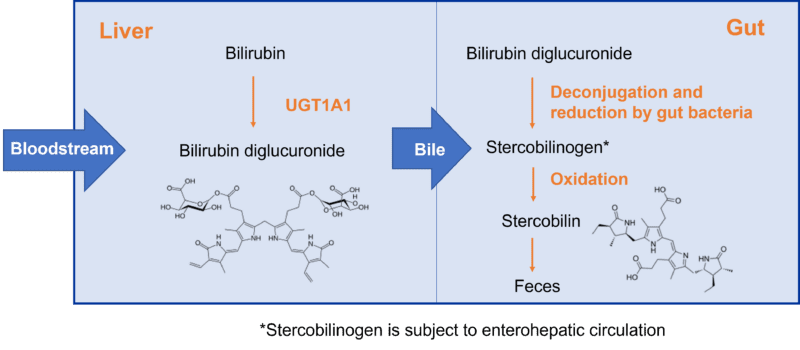
Figure 3: Simplified pathway to formation of stercobilin in the gut
The conjugation of inavolisib with stercobilin is observed in both live and heat-treated fecal incubations indicating a chemical reaction despite conjugation occurring to a higher extent in live feces. The authors propose that the conjugation proceeds by nucleophilic attack from the imidazole ring of inavolisib to the bridging carbon of a stercobilin molecule. The authors point out that stercobilin conjugation could continue to occur after sample collection, and thus efforts were made to develop a sample preparation method to minimize artificial generation of these metabolites.
Although stercobilin-derived drug metabolites have not been reported previously, the authors highlight the possibility of other drugs with electron-rich imidazoles or similar ring systems to undergo reactions with stercobilin.
References
[1] Hanan EJ, Braun MG, Heald RA, MacLeod C, Chan C, Clausen S, Edgar KA, Eigenbrot C, Elliott R, Endres N, Friedman LS, Gogol E, Gu XH, Thibodeau RH, Jackson PS, Kiefer JR, Knight JD, Nannini M, Narukulla R, Pace A, Pang J, Purkey HE, Salphati L, Sampath D, Schmidt S, Sideris S, Song K, Sujatha-Bhaskar S, Ultsch M, Wallweber H, Xin J, Yeap S, Young A, Zhong Y, Staben ST. Discovery of GDC-0077 (Inavolisib), a Highly Selective Inhibitor and Degrader of Mutant PI3Kα. J Med Chem. 2022 Dec 22;65(24):16589-16621. https://doi.org/10.1021/acs.jmedchem.2c01422
[2] Absorption, metabolism and excretion of inavolisib (GDC-0077), a PI3Kα inhibitor in humans. Sungjoon Cho, Shuguang Ma, Ryan Johnson, Sravanthi Cheeti, Cyrus Khojasteh, Donglu Zhang, Laurent Salphati. Drug Metabolism and Pharmacokinetics 55 Supplement, 2024, 100922. https://doi.org/10.1016/j.dmpk.2023.100922.
[3] Discovery of Unprecedented Human Stercobilin Conjugates. Sungjoon Cho, Lionel Cheruzel, Jingwei Cai, Stephen K. Wrigley, Renia T. Gemmell, Tetsuo Kokubun, Jonathan C. P. Steele, Laurent Salphati, Donglu Zhang and S. Cyrus Khojasteh. Drug Metabolism and Disposition July 11, 2024, DMD-AR-2024-001725; DOI: https://doi.org/10.1124/dmd.124.001725
[4] Hall, B., Levy, S., Dufault-Thompson, K. et al. BilR is a gut microbial enzyme that reduces bilirubin to urobilinogen. Nat Microbiol 9, 173–184 (2024). https://doi.org/10.1038/s41564-023-01549-x
[5] Sanada, S., Suzuki, T., Nagata, A. et al. Intestinal microbial metabolite stercobilin involvement in the chronic inflammation of ob/ob mice. Nature Sci Rep 10, 6479 (2020). https://doi.org/10.1038/s41598-020-63627-y