One-Stop Metabolite Shop
Chemical Synthesis
Chemical Synthesis of Drug Metabolites
We have developed a number of late-stage chemical synthesis methods for cost effective access to both phase 1 drug metabolites and phase 2 conjugates such as glucuronides, glycosides and sulfates. In addition to late-stage methods, we are also able to devise and execute synthetic or mixed synthetic-enzyme catalysed schemes for more challenging metabolites.
Chemical synthesis at Hypha complements the biosynthetic methods to provide a comprehensive service for clients to access all types of drug metabolites under the One-Stop Metabolite Shop umbrella.
A combination of internal chemical synthesis at Hypha, and project management of larger scale-up projects at our chemistry CRO partners, enables Hypha’s One-Stop Metabolite Shop to provide access to difficult-to-access metabolites from mg to kg-scale.
Late-stage chemical screens
Our late-stage chemical methods are effective for synthesis of all types of glucuronides, as well as oxidised, glucosidated and sulfated metabolites. Reactions are fully scalable and are a proven and cost-effective way to access metabolites synthesised using these methods.
Late-stage chemical screen for the synthesis of O-, N-, N-carbamoyl and acyl glucuronides
A proprietary screen consisting of multiple sets of robust and diverse conditions has been developed at Hypha for late-stage synthesis of O-, N– and acyl glucuronides of client drug compounds. Glucuronides are produced direct from the parent compound using tailored deprotection strategies compatible with acyl glucuronides and sensitive N-glucuronides.
Using this screen, the structure of the client’s parent drug compound does not need to be revealed. The methods are readily scalable and metabolites can be purified by chemists at Hypha.
We are also experts in purification, structure elucidation of the metabolites synthesised, and can provide Certificates of Analysis.
Late-stage oxidation screen
We have assembled a panel of late stage chemical oxidation conditions to screen for oxidised metabolites and degradation products of drugs.
The chemical oxidation screen creates oxidised products using 14 diverse conditions, constructed from a comprehensive evaluation of the literature and in-house knowledge. Reactions can be scaled to make gram quantities of the oxidised product.
We also have electrochemistry and photochemistry methods for making phase 1 metabolites and degradation products of drugs. Electrochemical oxidation examples include:
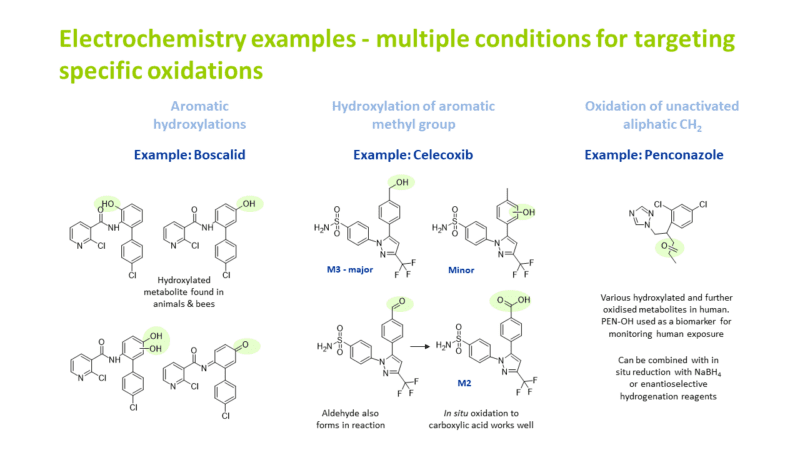
Hydroxylation at an unactivated CH2 position
Aromatic hydroxylation
α-hydroxylation next to an oxygen in a five membered ring system
Hydroxylation of an aromatic methyl group
Case Studies
Provision of N- and O-glucuronides of a client drug
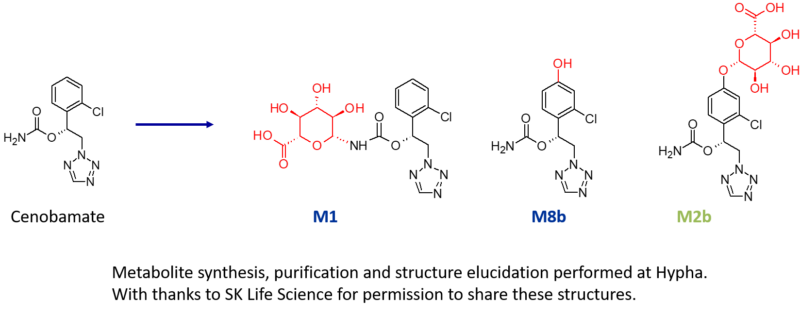
Three metabolites of cenobamate were required by SK Life Science; an N-glucuronide (M1), an indirect O-glucuronide (M2b) and a hydroxylated metabolite (M8b). As part of this project, multiple components of Hypha’s one-stop metabolite shop were employed, including chemical synthesis, microbial biotransformation as well as purification and structure elucidation by NMR.
M1, a major N-glucuronide, was accessed using chemical synthesis after identifying the best conditions in the chemical screens, resulting in the purification of a gram of M1. Key to successful synthesis were the mild deprotection conditions used in the late-stage chemical glucuronidation procedure.
In addition to the N-glucuronide, an indirect O-glucuronide, M2b, and its aglycone, M8b, were also needed. To make M8b, the position of the hydroxyl group first had to be identified. To achieve this, Hypha chemists purified a small amount of M2b from human urine supplied by the client, and elucidated the structure of the conjugate using cryoprobe NMR spectroscopy. Then, knowing the position of hydroxylation from the structure of the phenolic glucuronide, 100s of mgs of M8b were synthesised.
In order to access large amounts of M2b, a different approach was needed as this glucuronide was not amenable to chemical synthesis due to instability and formation of side products. Instead, M2b was successfully made through microbial biotransformation of the aglycone M8b. Following a screen to determine the best microbial catalyst, 560 mg of M8b was fed to one of Hypha’s biotransforming bacterial strains, from which 254 mg of M2b was purified. Metabolites were supplied to the client along with certificates of analysis.
Synthesis of two glucuronides of posaconazole
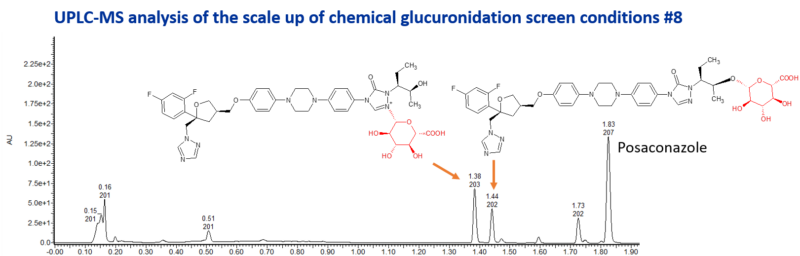 Posaconazole is a triazole antifungal drug which is slowly eliminated. It is a potent inhibitor of CYP3A4. It’s metabolism is mediated through glucuronidation by UGT1A4. A glucuronide of unknown structure, designated M8, is a major circulating metabolite in plasma, constituting up to 28% of the plasma radioactivity at 12 h postdose. Another glucuronide, M9, is a minor metabolite in plasma, where the sugar is attached at the 4″ position of the triazole ring. A diglucuronide, M5, has also been identified as a minor metabolite.
Posaconazole is a triazole antifungal drug which is slowly eliminated. It is a potent inhibitor of CYP3A4. It’s metabolism is mediated through glucuronidation by UGT1A4. A glucuronide of unknown structure, designated M8, is a major circulating metabolite in plasma, constituting up to 28% of the plasma radioactivity at 12 h postdose. Another glucuronide, M9, is a minor metabolite in plasma, where the sugar is attached at the 4″ position of the triazole ring. A diglucuronide, M5, has also been identified as a minor metabolite.
Late-stage chemical glucuronidation resulted in the formation of two major glucuronides and 1 other minor glucuronide. The major glucuronides were scaled up using the best chemical synthesis conditions. The metabolites were then purified for structure elucidation by NMR spectroscopy, resulting in the identification of an O-glucuronide and an N-glucuronide.
Development of an alternative late-stage method for an unstable acyl glucuronide
A European pharma client required 5-10 mgs of an acyl glucuronide. The drug compound was initially profiled in the standard chemical glucuronidation and microbial biotransformation screens. However, we encountered some challenges in production of acyl migration isomers via the microbial route, and low yields due to extensive hydrolysis back to the parent drug in the four chemical glucuronidation methods. The problem was quickly solved by development of an alternative two-step late-stage chemical synthesis route which was more suitable for this hydrolytically-unstable acyl glucuronide, to achieve a 30% isolatable yield of the target acyl glucuronide. After purification and confirmation of the structure by NMR, 30.5 mg was delivered to the client together with a certificate of analysis.
Late-stage chemical sulfation of an intermediate drug metabolite
A sulfated metabolite was required by a US client which was produced in humans through an aryl hydroxylated intermediate. Hypha used microbial biotransformation to produce the intermediate metabolite followed by late-stage chemical synthesis to make and purify hundreds of milligrams of both unlabelled and a deuterated O-sulfated metabolite.
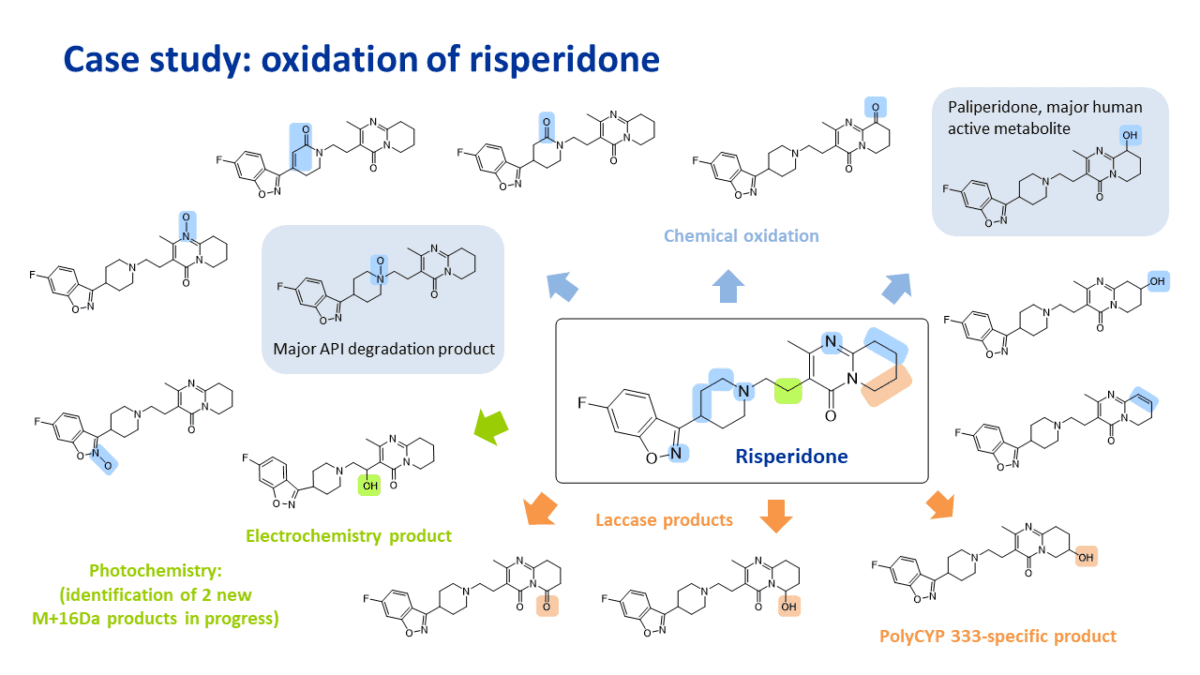
Late-stage oxidation of risperidone
Application of a panel of oxidation conditions resulted in the synthesis and identification of 14 oxidation products of risperidone, including the formation of 9-hydroxy risperidone, also known as paliperidone, the primary active metabolite of risperidone (circled in blue below).
Oxidation conditions applied include a range of chemical reagents, electrochemistry and photochemistry.
Some of the oxidation conditions also provide a route to obtain products typically formed in API degradation studies, such as the main N-oxide of risperidone (circled in blue below).
Our other one-stop shop solutions for accessing and characterising metabolites
Hypha’s One-Stop Metabolite Shop enables synthesis, purification and characterization of all the main types of mammalian phase 1 and 2 metabolites.
Mammalian biotransformation
Panels of liver S9s / microsomes
Recombinant enzymes
PolyCYPs, hrCYPs, AOX, FMOs
Microbial biotransformation
Proven panels of bacteria and fungi
Purification & structure elucidation
COAs, acquisition / interpretation of NMR data
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
Access our brochure on Hypha’s One-Stop Metabolite Shop, which describes how we synthesise, purify and characterise all the main types of mammalian phase 1 and 2 metabolites.
We’ve observed an increase in requests for synthesis of N-glucuronides over the last couple of years. We speculate that this may be due to the increasing use of N-heterocyclic chemistry in the design of new small molecule drugs, and pan company strategies to reduce CYP metabolism. The situation is further complicated by the high interspecies variability in formation of some N-glucuronides, especially aliphatic tertiary amines and aromatic N-heterocycles. UGT1A4 and UGT2B10 are key enzymes responsible for N-glucuronidation reactions in humans, rates of which can be much higher than in other animals. To compound this, synthesis of N-glucuronides is not always straightforward, and can be further muddied by metabolite stability issues, complicating interpretation of data.
Access to multiple metabolites needed to support clinical development is not always straightforward, and can sometimes mean that more than one technique needs to be applied to fulfil requirements. In one such project, a US pharma client required > 200 mg of three metabolites of a drug; an N-glucuronide (M1), an indirect O-glucuronide (M2b) and a hydroxylated metabolite (M8b). As part of this project, multiple components of Hypha’s one-stop metabolite shop were employed, including chemical synthesis, microbial biotransformation as well as purification and structure elucidation by NMR.

We worked with Hypha discovery to synthesize metabolites via a chemical approach in enough quantities for pharmacology studies for one of our products. Hypha has well understood our request and has provided a very good quality of service in time, in quantity and quality with the needed level of regular communication. We will work certainly with them in the future and will recommend them.
Dr. Laure Navarre, Director Process Chemistry
Poxel, France
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk


