One-Stop Metabolite Shop
Microbial Biotransformation
Metabolite Synthesis using Microbial Biotransformation
For difficult-to-synthesise metabolites, we employ our talented microorganism panels to create metabolites of drugs and agrochemicals. Microbial biotransformation will produce most mammalian metabolites as it is well-established that microbial organisms mimic mammalian metabolism of small molecules due to evolutionarily-related enzyme systems. The relevance of the technique to agrochemical metabolism is also established, due largely to the representation of soil microbes in our strain panels. The microbial route provides a cost-efficient method for scaling mg to gram scale amounts, as the microbes make their own co-factors.
As a natural extension to our work with microbes, we’ve recently developed an anaerobic screen for gut metabolites using pooled human faeces. This technique is suitable for generation of microgram to milligram amounts needed for MetID and biological testing.
Microbial biotransformation is highly effective for making many human and other mammalian metabolites
Case Studies
Production of a human disproportionate hydroxylated metabolite
Ingenol disoxate is a chemically-stable topical dermal clinical drug candidate that was being developed by one of Hypha’s clients for treating actinic keratosis. Profiling of ingenol disoxate against multiple species of hepatocytes, revealed M27 as a predominant metabolite, particularly in human hepatocytes. Although accurate mass spectroscopy indicated the metabolite was mono-hydroxylated in the ingenol moiety, the precise location of the hydroxyl group could not be identified. Consequently, chemical synthesis was not feasible, nor were bioanalytical quantification and further biological testing possible. Hypha subsequently solved these issues for the client using microbial biotransformation.
Hypha’s microbial screen highlighted fourteen strains that produced oxidised metabolites of ingenol disoxate. After precise chromatographic matching by the client, the best-yielding strain was scaled up to produce a target amount of 10-50 mg of the human metabolite M27.
Following scale-up, 86 mg of M27 was supplied to the client at >99% purity, who identified the metabolite as 16-hydroxyingenol disoxate. In addition to this material, 5 mg of a dihydroxylated derivative was supplied for evaluation. A subsequent request for more M27 for further studies needed a minimum of 200 mg, with Hypha supplying 415 mg of M27 to the client at 99% purity.
Having plentiful supply of this material meant that scientists were able to confirm the structure of the major metabolite via NMR spectroscopy and undertake PK profiling assays, as well as respond immediately to a later request by the FDA for drug-drug interaction studies to be undertaken with the metabolite.
Reference
Carlsen et al., 2016. Biosynthesis, structural identification and quantification of low pg/ml levels of a major human metabolite of a dermal drug candidate. European Bioanalysis Forum, Barcelona, Nov. 2016.
Production of metabolites of epacadostat arising from multiple pathways
Formation and scale-up of human metabolites formed through mixed metabolic pathways is possible using Hypha’s microbial biotransformation system.
In vivo human metabolism of epacadostat (EPA) forms 3 major circulating metabolites, from both primary and secondary pathways. Glucuronidation of EPA forms M9, the dominant metabolic pathway, in conjunction with formation of an amidine M11 and an N-dealkylated metabolite, M12. Boer et al. showed reductive metabolism by gut microbiota results in M11, which is absorbed and further modified by CYP enzymes to form the cleaved secondary metabolite M12.
Hypha’s microbial panels provided a route to achieve formation of all three human metabolites, with several strains shown able to effectively biotransform EPA. Different strains and dosing regimens were found to be optimal for production of each metabolite. Although M12 was produced by the microbes, it was more easily synthesised.
Scale-up of the most productive biotransforming strains for M9 and M11 enabled the supply of 112mg of the glucuronide and 69mg of the gut metabolite at 95% purity to the client, which permitted the qualification of the metabolites for their drug-drug interaction potential.
Our other solutions for accessing and characterising metabolites
Hypha’s One-Stop Metabolite Shop enables synthesis, purification and characterization of all the main types of mammalian phase 1 and 2 metabolites.
Chemical Synthesis
Late-stage chemical glucuronidation
Recombinant enzymes
PolyCYPs, AO, FMOs, GTs
Gut metabolites
Anaerobic human faecal incubations
Mammalian biotransformation
Panels of liver S9s / microsomes
Purification & structure elucidation
COAs, acquisition / interpretation of NMR data
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do
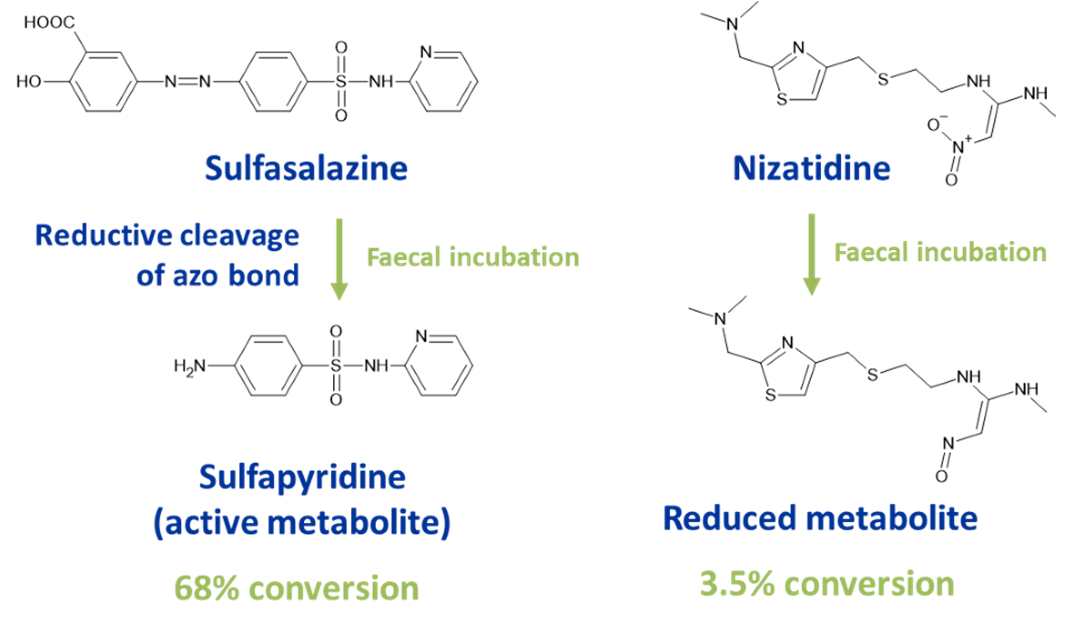
Hypha’s human faecal pool can be used to screen for reduced metabolites of drugs, as exemplified with sulfasalazine and nizatidine.
In this case study, all of the main human metabolites of cyclosporin A were produced using microbes in Hypha’s biotransformation panels, and by using PolyCYPs® enzymes. Additionally we are able to form novel microbial-derived metabolites.
Metabolite identification is an integral part of both preclinical and clinical drug discovery and development. Synthesis of drug metabolites is often required to support definitive identification, preclinical safety studies and clinical trials.

Hypha Discovery has been a valuable metabolite ID partner. Hypha have provided biotransformation, metabolite purification and structure elucidation answers to some of our most challenging metabolism and metabolite ID problems. We really appreciate the breadth of expertise available at Hypha Discovery and will definitely reach out for future work.
Director of DMPK
US Pharma Company
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk