What We Do
MetID Services
LC-MS/MS MetID Services
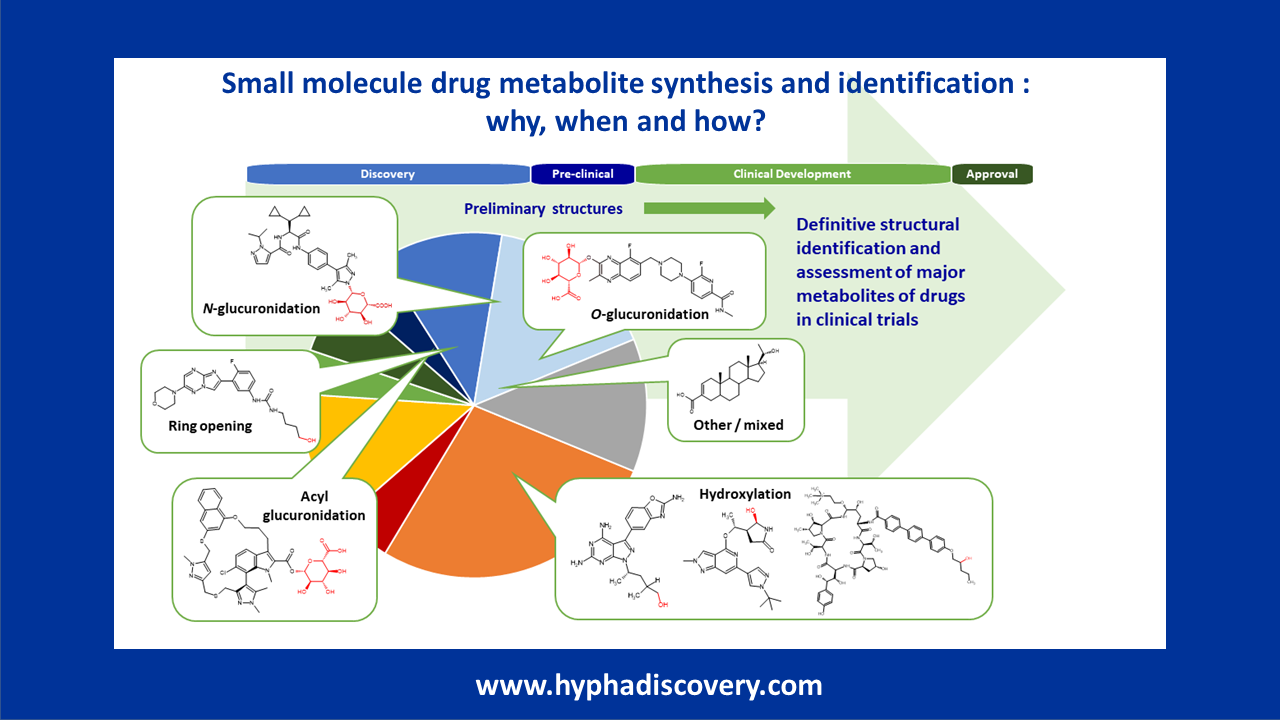
Hypha’s expertise spans a full range of services for confident MetID. From identifying metabolic soft spots of discovery stage compounds to conducting more in depth biotransformation studies for lead compound selection and beyond, our capabilities support critical stages of drug development.
Hypha offers a range of MetID packages tailored to client requirements. We can generate metabolites using a variety of multi species in vitro systems such as hepatocytes and microsomes, including use of methods suitable for slowly metabolised compounds.
We utilise a Thermo Scientific Vanquish Horizon UHPLC and Orbitrap ExplorisTM 120 LC-MS/MS system, which enables the detection and identification of a wide spectrum of both expected and unexpected metabolites, including those present at low abundance. Its sub-ppm mass accuracy and high resolution allow for differentiation of isobaric compounds, providing high confidence in metabolite identification.
We also routinely provide NMR spectroscopy based definitive MetID for those metabolites where knowledge of the full structure is important. We can utilise either a 600 MHz machine, or a 700 MHz machine fitted with a 1.7mm cryoprobe to reduce the amount of pure metabolite needed for full data acquisition to support structure elucidation. Interpretation and reporting of the data is done by experts at Hypha.
What We Do
Introductory screening offer
To help you get the most from your MetID study, we’re offering free screening of your compound using our PolyCYPs and PolyUGTs metabolite synthesis kits for 6 months after delivery of your MetID report.
This exclusive offer for Hypha’s MetID clients facilitates metabolite generation for definitive structure elucidation, and helps accelerate downstream biological testing, or where metabolite standards are needed.

Key features
Multi species in vitro panels
Biofluids and tissues from in vivo studies, e.g. plasma, urine
Metabolite profiling and ID by LC-MS/MS
Multiple scalable biotransformation and chemical synthesis methods for accessing Phase I, Phase II and mixed phase metabolites
Definitive structure elucidation by cryoprobe NMR spectroscopy and purity assessment by qNMR for bioanalysis standards
Hepatocytes, microsomes, S9 etc.
Anaerobic faecal incubation system (human and rat) for identification of gut-derived metabolites
HµREL® and HEPATOPAC® coculture systems (hepatocytes and stromal cells) for slowly metabolised compounds
Metabolite purification, stability testing and Certificates of Analysis
Synthesis of stable-labelled metabolites
MetID packages
Identification of metabolism pathways driving clearance is a critical component in understanding metabolic liabilities of your drug candidate, and to assess the suitability of preclinical animal tox models. Importantly, any human specific or disproportionate metabolites should be understood at an early a stage as possible. Further, the drug-drug interaction potential of specific metabolites may need to be understood, along with the effect of any active metabolites on PK.
Work programs can be tailored to fit your requirements around our main packages. We can work with various incubation formats such as hepatocyte suspension assays, microsomal fractions or recombinant enzymes. For more slowly metabolised compounds, we can utilise the HEPATOPAC® or HµREL® micro liver coculture systems which allows longer term incubations of 72h.
Contact us to discuss your specific requirements.
Case study – MetID of repaglinide
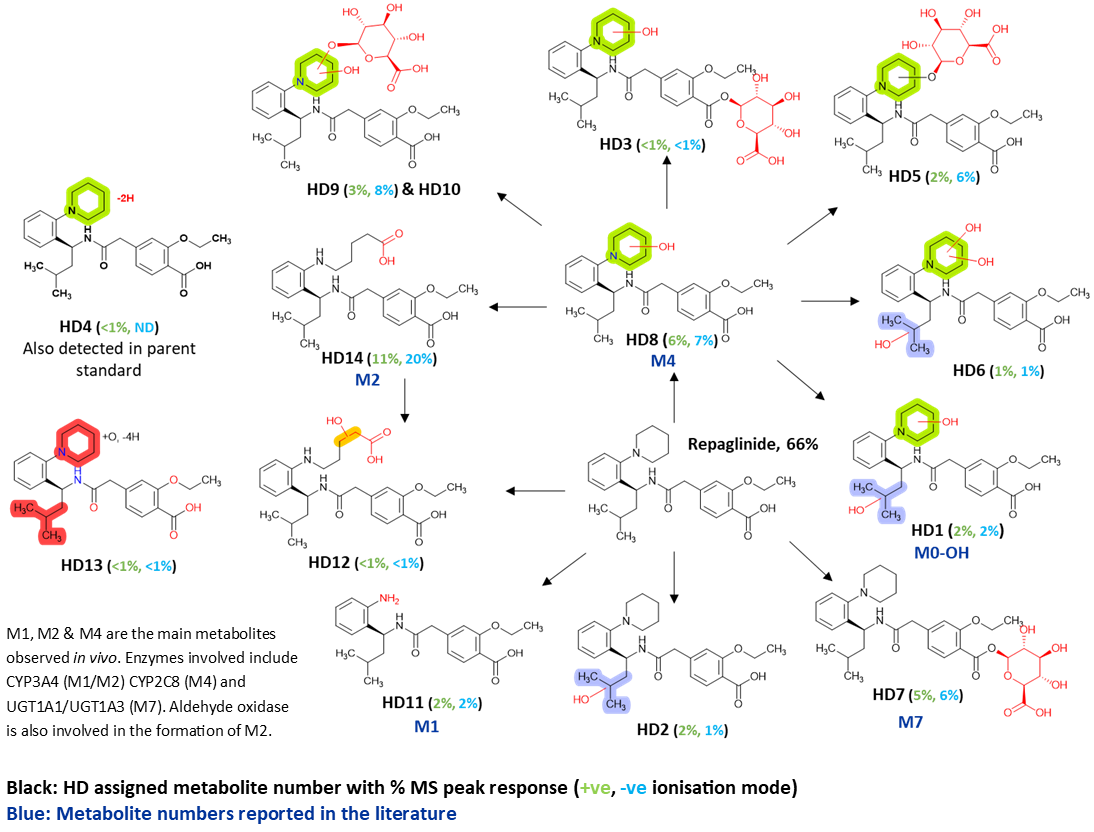
Metabolism of repaglinide (5 µM) was studied in human and rat cryopreserved hepatocytes (1 million cells/mL) at 37oC for 120 mins, with subsequent analysis by LC-MS/MS in positive and negative ionisation modes. Metabolites observed were compared to those reported in the literature, confirming production in Hypha’s incubation system. In addition, potentially new metabolites were detected that have not been previously published.
In human hepatocytes, 14 metabolites were detected. The 3 main in vitro metabolites reported by Gan et al. (M2, M4 and the glucuronide M7) corresponded to the top 3 metabolites (HD14, HD8, HD7) observed in this study. Ten other metabolites were annotated, arising from oxidation of the aliphatic side chain, oxidative dehydrogenation, further oxidations and secondary glucuronidation. Only 6 of the human metabolites were detected in rat hepatocyte incubations (data not shown), including M2 and M7 but not M4. The de-ethylated metabolite M5 was only seen in rat hepatocytes.
Incubation of repaglinide with PolyCYPs and PolyUGTs reproduced the major metabolites produced in human hepatocytes (CYP derived metabolites M0-OH, M1, M2, M4, and the acyl glucuronide M7). Max % conversions observed in the screen (based on MS peak area in +ve ionisation mode) were all in the scalable range; M0-OH 7.6% (PolyCYP 359), M1 83% (PolyCYP 359), M2 39% (PolyCYP 486), M4 42% (PolyCYP 349) and M7 21% (PolyUGT 194).

References
Repaglinide-gemfibrozil drug interaction: inhibition of repaglinide glucuronidation as a potential additional contributing mechanism. Gan et al. Br. J. Clin. Pharmacol. 2010; 70(6): 870-880. https://doi.org/10.1111/j.1365-2125.2010.03772.x
A Comprehensive Assessment of Repaglinide Metabolic Pathways: Impact of Choice of In Vitro System and Relative Enzyme Contribution to In Vitro Clearance. Säll et al., Drug Metab. Dispos. 2012; 40(7): 1279-1289. https://doi.org/10.1124/dmd.112.045286
Routes to accessing metabolites for definitive MetID
Users of Hypha’s MetID service benefit from Hypha’s world-leading metabolite synthesis services. In addition to providing the LC-MS/MS MetID data, we can make and purify metabolites for further testing or for use as reference standards using our proven scalable methods.
Techniques encompass the use of scalable surrogate biotransformation systems such as microbes and recombinant enzymes, as well as proprietary late-stage chemical methods. In addition, gut metabolites can be accessed using our human and rat faecal incubation systems.
For definitive MetID we elucidate structures using NMR spectroscopy. Typically only tens to 200 micrograms are needed for acquiring full data sets using a 700 MHz machine in conjunction with a 1.7mm micro-cryoprobe. We also routinely use quantitative NMR to assess the purity of the metabolites we produce.

Example
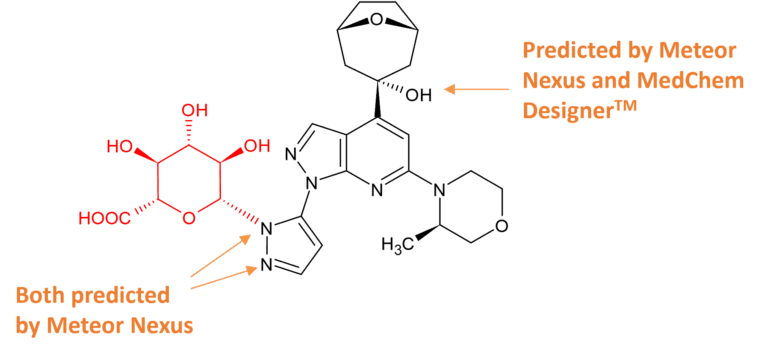
The position of glucuronidation of camonsertib was not possible to assign using LC-MS/MS or prediction software. Microbial biotransformation was used to scale-up the UGT-1A4 derived metabolite to provide material for structure elucidation by NMR spectroscopy, stability studies, and for use as a standard for bioanalysis.
Ref: Papp et al. N-Glucuronide Metabolite of Camonsertib. Drug Metabolism and Disposition , 2024, 52(5): 368-376. https://doi.org/10.1124/dmd.123.001611
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
Access our brochure on Hypha’s One-Stop Metabolite Services and Kits to discover routes for synthesising all the main types of mammalian phase I and II metabolites.
Download a brochure on Hypha’s MetID services spanning LC-MS/MS metabolite profiling to definitive structure elucidation by NMR spectroscopy.
In this open access review in Drug Discovery Today we look at the why, when and how metabolites of small-molecule drugs are synthesised from the perspective of a specialist CRO.
Find out about our other services and products

Hypha's team did an excellent job by synthesizing the glucuronide for our project. They obtained the right structure which was not the one expected (as several options were possible). Sometimes, LC/MS-MS fragmentation do not deliver the right results. Hypha corrected the structure after NMR investigation. Now we have this important glucuronide for us with the right structure and have the good answer for the project. We really appreciated the hard work and the flexibility of the team to deliver the material.
Phillipe Guedat, CEO
Inflectis BioScience, France
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Supplier Code of Conduct Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved.