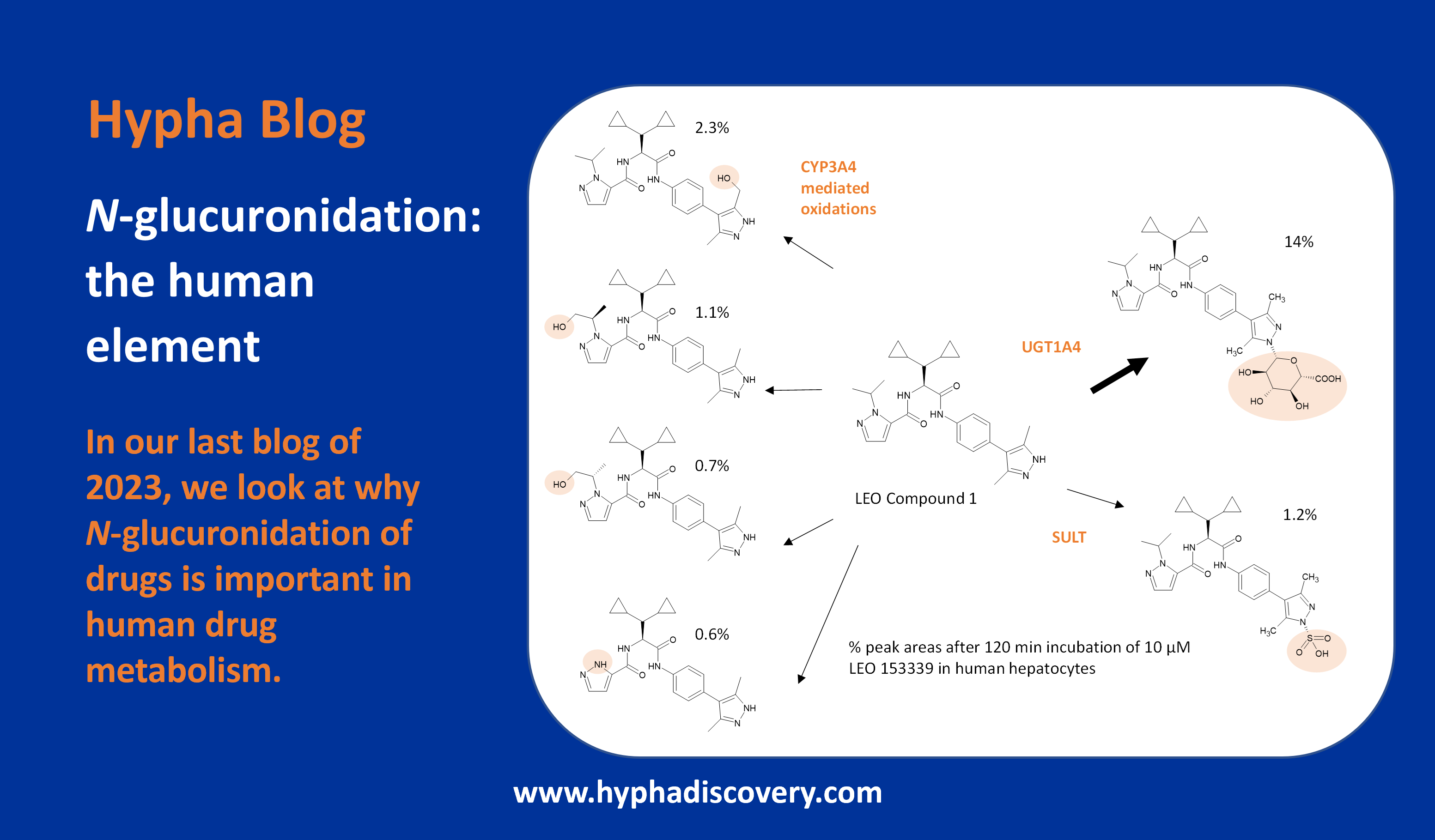
De-risking DMPK: what’s key?
By Guy Webber
Drugs can fail i) before they enter clinical development in humans ii) during clinical trials or iii) after clinical trials and approval. In many ways, it is better they fail early, before patient exposure and costly clinical trials, with new drug approvals running at over an average $2billion per approved drug.
Clinical failure due to lack of efficacy is very difficult to predict and the failure to transit from Phase II to Phase III is still a major cause of drug attrition today.
But what about before and after clinical development? Can we better identify the risks here?
The answer depends on understanding what some of the major pre- and post-clinical risks are. And in particular, what drives those risks. If we exclude lack of clinical efficacy, the major risk driver for a new [small molecule] chemical entity will often be driven by its DMPK. In other words, how the new molecule is metabolised and distributed/transported in humans.
Drug metabolism is pivotal for small molecule drug development – if you have ‘atypical metabolism’ you will face certain challenges as the process of drug metabolism itself drives 3 substantial risks: i) having a high DDI potential, ii) producing human-specific metabolites and iii) causing idiosyncratic DILI.
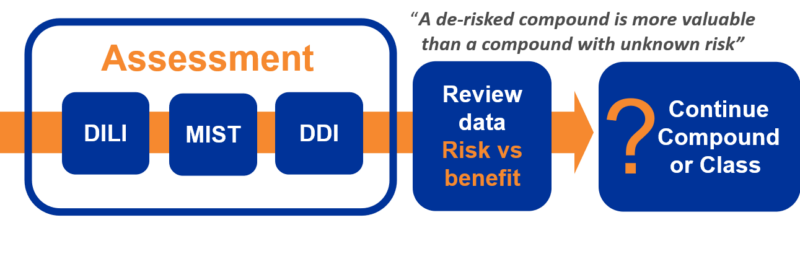
What risks do we need to worry about?
Looking at each of these briefly in turn, what are the DDI issues to be concerned about? Is it the new molecule causing inhibition of drug metabolising enzymes or drug transporter proteins? In other words, is it the new molecule acting as a perpetrator of drug-drug interactions?
Actually, no. This is not the major DDI risk. The major risk of non-approval is greater as a victim drug. In this scenario, there is often only a single enzyme or transporter dominating the molecule’s clearance, and if the fraction metabolised (Fm) or fraction transported (Ft) by an individual enzyme or transporter, is too high, then unless the molecule has a wide therapeutic window or meets a currently unmet medical need (risk vs benefit), there is risk of non-approval. Examples of drug non-approvals due to a high victim drug DDI status include debrisoquine and perhexiline and post-market withdrawals include terfenadine, cerivastatin, astemizole and cisapride. As an overall want, it is better to have multiple enzymes/transporters mediating drug clearance in case one of these pathways are inhibited by a concomitant medication. This information is provided by in vitro assays known as reaction phenotyping studies, which use human liver microsomes, hepatocytes and recombinant transporter expressing cells to map and quantify the clearance mechanisms and pathways (enzymes and transporters) in humans.
And what happens if your molecule generates a unique metabolite in humans? This could mean your safety assessment data in animals cannot be fully extrapolated to humans. This is a major concern of regulatory bodies like the FDA as exemplified by their recent guidance for industry in this area (FDA Safety Testing Of Metabolites guidance of 2020, often known as MIST). Although true human-unique [plasma] metabolites are actually quite rare, if one is present at >10% total drug related material, it might be required to synthesise the metabolite and conduct additional toxicological assessments. Early hepatocyte metabolite profiling studies in combination with animal in vivo profiling (plasma, urine) can help build a data set which can alert potential human-specific metabolism.
And finally, still the number 1 cause of safety-related drug withdrawal for small molecule drugs post-market approval remains human DILI, or drug-induced-liver-injury. To fail after development and approval is catastrophic for patient safety and for the company involved and for the industry as a whole. Drug withdrawals do not make good worldwide headlines. Drug metabolism is key to understanding DILI liability and there is a marked need to identify DILI-positive compounds during discovery and lead optimisation.
Fortunately, advancements in modern in vitro technologies and analytical instrumentation allows DMPK scientists to apply a variety of in vitro based assays designed to rapidly assess risk-liability to these potential red flags which contribute to attrition rates and delays/stalls in development and screen out potential high-risk candidates from selection and development.
The ready availability of characterised sub-cellular and cellular models and the low compound requirements typically required for in vitro assays facilitates the early de-risking assays to be enrolled during the discovery phase.
Don’t put all your eggs in one basket
It is important to factor in to an early metabolic de-risking strategy that individual in vitro assays, may (sometimes) not by themselves, be predictive and the reliance of a single assay for DILI for example, such as covalent binding only, runs the risk of not fully identifying the risk potential. Therefore, to maximise the unearthing of ‘red flags’ a multi-parameter (assay) approach is recommended combining multiple signals including covalent binding, reactive metabolite trapping and CYP TDI to better assess the DILI risk (in combination with early dose to man predictions). The concept of early de-risking can be seen as ‘value-adding’ as a de-risked molecule is more valuable in many ways than a molecule whose metabolic risk liability status in unknown (other developmental factors being like for like) and can aid out-licensing.
The primary aim of an early de-risking strategy is to provide a comprehensive and rigorous data set, which allows a rational, scientifically-based assessment to be made about the likelihood that a molecule may carry a liability, before major investment decisions are made. With this in mind, integrating metabolic de-risking into drug discovery would seem to be an advisable strategy given that the pharma industry operates in an increasingly risk-averse environment.
Guy Webber
Director, DMPK Services Ltd.
Preclinical DMPK, DDI and IND Consultancy
References
- Cook, D., Brown, D., Alexander, R. et al. Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat Rev Drug Discov 13, 419–431 (2014). https://doi.org/10.1038/nrd4309
- Drug development: the journey of a medicine from lab to shelf by Ingrid Torjesen. Tomorrow’s Pharmacist, May 2015; DOI:10.1211/PJ.2021.1.71304
- Pharma Derisking: Approaches to Reduce Liability to Major Metabolically-Driven Causes of Drug Attrition. Drug Discovery World, April 2017
- Structural alert/reactive metabolite concept as applied in medicinal chemistry to mitigate the risk of idiosyncratic drug toxicity: a perspective based on the critical examination of trends in the top 200 drugs marketed in the United States. Stepan AF, Walker DP, Bauman J, Price DA, Baillie TA, Kalgutkar AS, Aleo MD. Chem. Res. Toxicol. 2011, 24, 9, 1345–1410. https://pubs.acs.org/doi/10.1021/tx200168d
- In Vitro Drug Interaction Studies — Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry, Docket Number: FDA-2017-D-5961, Center for Drug Evaluation and Research FDA, JANUARY 2020
Abbreviations
DMPK = Drug metabolism and pharmacokinetics
DDI = Drug-drug Interactions
DILI = Drug Induced Liver Injury
CYP = Cytochrome P450
TDI = Time-dependent Inhibition
MIST = Metabolites In Safety Testing
NDA = New Drug Application