Metabolism of 2023 FDA Approved Small Molecules – PART 2
A focus on drugs where metabolism in humans results in circulating glucuronides and other conjugates
By Julia Shanu-Wilson
Following on from our first delve into the metabolism of small molecule drugs approved last year where active metabolites were reported, we now look at a subset of drugs where phase II mechanisms are involved. The primary route of metabolism of many of these also involve cytochrome P450 enzymes. Where data was publicly available, the routes of human metabolism for each of the drugs in this subset is listed in Table 1 .
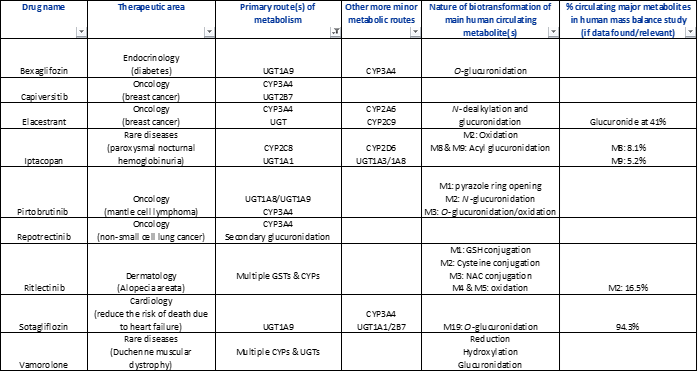
Table 1: Small molecule drugs approved by the FDA in 2023 with reported involvement of phase II mechanisms
In vitro : In vivo differences
Incubation of the SGLT2 (sodium-glucose co-transporter-2) inhibitor bexagliflozin in human liver microsomes points to metabolism through both oxidation and glucuronidation to 6 main metabolites. The most prominent oxidative metabolites are formed by the action of CYP3A4 through cleavage of the cyclopropyl ether and by ether cleavage followed by oxidation of the resulting primary alcohol to a carboxylic acid, or by direct formation of the carboxylic acid [1].
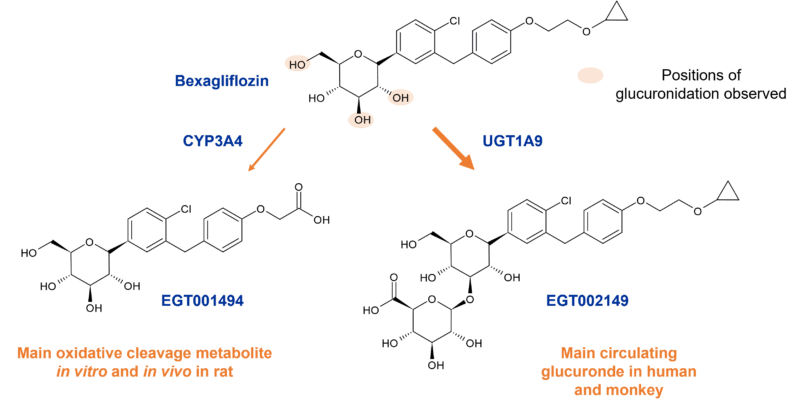
Figure 1: Main circulating metabolites of bexagliflozin in human, monkey and rat
However, a different story becomes apparent in vivo where a greater diversity of metabolites is evident from studies in human, and with one of three O-glucuronides (EGT0002149) dominating through the action of UGT1A9. This in vivo difference may partly be due to the higher expression of UGT1A9 in kidneys compared to the liver. The 3’-O-glucuronide (EGT002149) contributes 32% of the parent AUC with other metabolites comprising < 10% following oral dosing in humans. Data suggests that microbial glucuronidases in the gut may cause degradation of EGT002149 and other glucuronides back to the parent drug, the action of which is however not proposed to contribute to plasma persistence via colonic absorption/re-absorption.
Metabolism in monkey most closely resembled human metabolism compared to other species such as rat where oxidation dominates. Metabolites found in humans are also observed in monkeys, and all metabolites were found to possess <10% of the activity of the parent drug.
Rather more straightforward is the biotransformation of sotagliflozin, another sodium-glucose co-transporter inhibitor (dual SGLT2/1), which is extensively metabolised to a major O-glucuronide (M19), also by UGT1A9. In the 14C radiolabelling study, M19 represented 94% of the dose in plasma. DDI studies undertaken with M19 showed that it is an inducer and inhibitor of CYP3A4 and an inhibitor of CYP2D6. The glucuronide also inhibited OATP1B1/B3 and MRP2 in vitro.
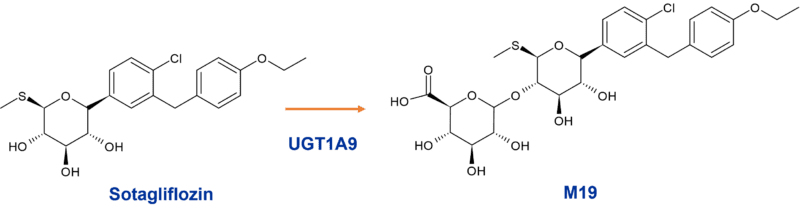
Figure 2: Major O-glucuronide M19 of sotagliflozin
Species differences in N-glucuronidation
The highly selective non-covalent inhibitor of Bruton’s tyrosine kinase (BTK) pirtobrutinib is another drug mainly metabolised by CYP3A4 and direct glucuronidation, the latter via UGT1A8 and UGT1A9. Biotransformations observed in human hepatocytes include hydroxylation, N-glucuronidation, and secondary O-glucuronidation following either O-demethylation or hydroxylation [2].
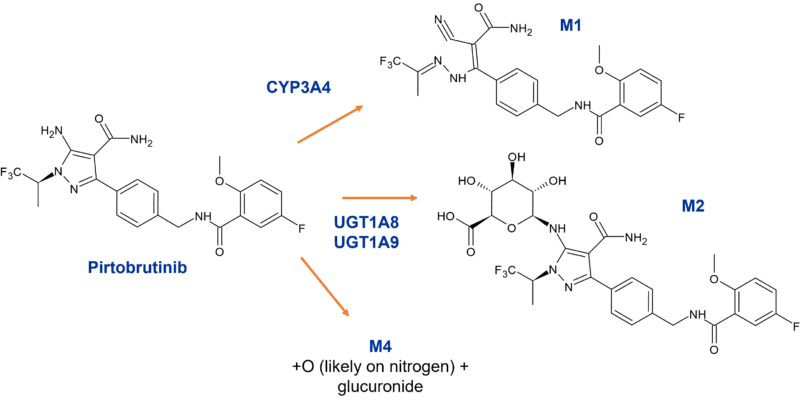
Figure 3: Metabolites of pirtobrutinib circulating in human plasma
Interestingly in the rat study, an oxidation pathway forming M1 and onward oxidation to M5 dominated in males comprising 33.9% and 20.1% respectively of total radioactivity vs 17.9% of parent. In contrast, in female rats the maximum individual metabolite circulated at less than < 5.6% of dose. In the human study M1 was also observed, contributing 7.8% of total circulating radioactivity. However, M2 (N-glucuronide) and M4 (monooxy N-glucuronide) were also observed as circulating metabolites at 3.3% and 2.3% of dose respectively. M1 was covered in rat, with appropriate steady state coverage in the rat 3-month toxicology study [3]. Since M2 and M4 comprised < 10% of total circulating radioactivity are glucuronides, no MIST related evaluations were undertaken on these metabolites.
Acyl glucuronidation
Iptacopan is metabolised through both oxidation and glucuronidation with eight metabolites accounting for 51.8% of the administered radiolabel in the human ADME study. Two circulating acyl glucuronides are observed in humans.
The acyl glucuronides are formed by UGT1A1 via direct glucuronidation of the parent drug to M8 (8.1% of plasma 14C-AUC0-48h) and secondary glucuronidation via M2 to M9 (5.2% of plasma 14C-AUC0-48h). CYP2C8 is responsible for O-deethylation to M2. None of the metabolites are pharmacologically active [5,6].
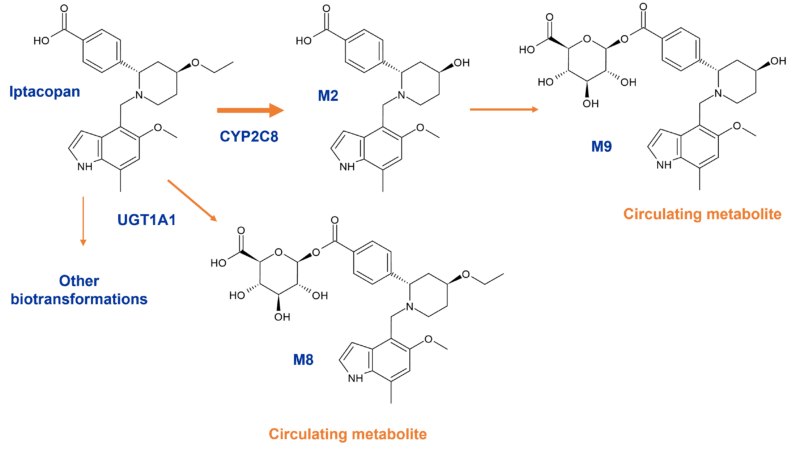
Figure 4: Circulating acyl glucuronides of iptacopan in humans
Both acyl glucuronides were also observed in the first-in-human phase 1 dose-finding study following multiple doses of oral iptacopan. In toxicity studies, exposure to the acyl glucuronide metabolite M8 at the no-observed– adverse-effect level was similar to or higher than human exposure at the highest phase 3 dose (200 mg twice daily) although exposure to the minor circulating metabolite M9 was lower. Based on studies conducted it was proposed that there was a low risk associated with the circulating acyl glucuronides.
Although stabilised in acidified plasma for analysis, half-lives of M8 and M9 in phosphate buffer at pH7.4 are 1.6 and 3 hours respectively. Some hydrolysis of M8 to the parent drug is observed under these conditions.
Circulating cysteine conjugates
Whilst no single route contributes to more than 25% of the total metabolism of ritlecitinib involving multiple CYPs (CYP 3A4, CYP2C8, CYP1A2, CYP2C9), more interesting is its metabolism by cytosolic and microsomal GSTs (glutathione-S-transferases) [7].
Several conjugates circulate in humans; the main circulating metabolites reported from one trial (oral 400mg/day dose over 14 days) include a glutathione-related metabolite (M1), a cysteine conjugate (M2) and an N-acetylcysteine conjugate (M3). Similar or higher metabolite levels were observed in rodents. The cysteine conjugate M2 represented 16.5% of circulating dose in the radiolabelled human ADME study (pooled, 0-48h) [9].
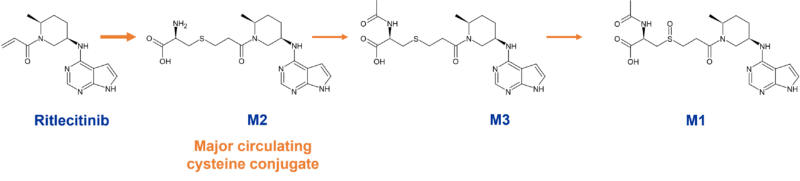
Figure 5: Circulating GST metabolites of ritlecitinib
Relevant to clearance via glutathione is ritlecitinib’s mechanism of action. Ritlecitinib is a covalent drug which irreversibly inhibits Janus kinase 3 (JAK3). It is approved for treatment of severe alopecia areata in which inflammatory processes play a major role, with associated increased reactive oxygen species formation and reduced levels of GSH. It is proposed that the decreased clearance of the drug observed in patients may be due to depletion of GSH in this population [10].
The next blog will complete our commentary on metabolism of small molecule drugs approved in 2023.
Note that where Hypha may have been involved in any of the projects described, no details on metabolites other than what is publicly available have been disclosed in this article.
References
[1] Zhang W, Li X, Ding H, Lu Y, Stilwell GE, Halvorsen YD, Welihinda A. Metabolism and disposition of the SGLT2 inhibitor bexagliflozin in rats, monkeys and humans. Xenobiotica. 2020; 50(5): 559-569. https://doi.org/10.1080/00498254.2019.1654634
[2] EMA Assessment Report for Jaypirca (pirtobrutinib). https://www.ema.europa.eu/en/documents/assessment-report/jaypirca-epar-public-assessment-report_en.pdf
[3] FDA Review application number 216059Orig1s000 for Jaypirca (pirtobrutinib (Loxo-305)). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/216059Orig1s000MultidisciplineR.pdf
[4] FDA prescribing information for INPEFATM (sotagliflozin). https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216203s000lbl.pdf
[5] James AD, Kulmatycki K, Poller B, Romeo AA, Van Lier JJ, Klein K, Pearson D. Absorption, Distribution, Metabolism, and Excretion of [14C]iptacopan in Healthy Male Volunteers and in In Vivo and In Vitro Studies. Drug Metab Dispos. 2023; 51(7):873-883. https://doi.org/10.1124/dmd.123.001290
[6] FDA prescribing information for FABHALTA® (iptacopan). https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/218276s000lbl.pdf
[7] FDA prescribing information for LITFULOTMTM (ritlecitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215830s000lbl.pdf
[9] Multi-disciplinary Review and Evaluation for NDA 215830 Litfulo (ritlecitinib) capsule. https://www.fda.gov/media/172964/download?attachment
[10] Wojciechowski J, S Purohit V, Huh Y, Banfield C, Nicholas T. Evolution of Ritlecitinib Population Pharmacokinetic Models During Clinical Drug Development. Clin Pharmacokinet. 2023; 62(12):1765-1779. https://doi.org/10.1007/s40262-023-01318-3