Bioisosteres for carboxylic acid groups
Carboxylic acid containing drugs – opportunities for substituting bioisosteres to mitigate the formation of acyl glucuronides
Our highlighted paper for April 2024 discusses the reasons and strategies for replacing a carboxylic acid with a bioisostere. Of particular interest is the authors use of structural biology to explore neutral bioisosteres that rely on less commonly explored cation π-interactions.
Why replace a carboxylic acid with a bioisostere? The authors highlight a few reasons including reducing the risk of forming reactive metabolites due to acyl glucuronidation.
Despite not all acyl glucuronides being reactive, there may be some instances where swapping in another moiety for a carboxylic acid may be a useful strategy to explore. In this paper the authors discuss various options including various negative ionizable groups described in the literature, and using structural biology to explore the possibility of substituting neutral bioisosteric groups. The latter strategy may be more effective for improving distribution to the CNS due to the lack of ionization at physiological pH.
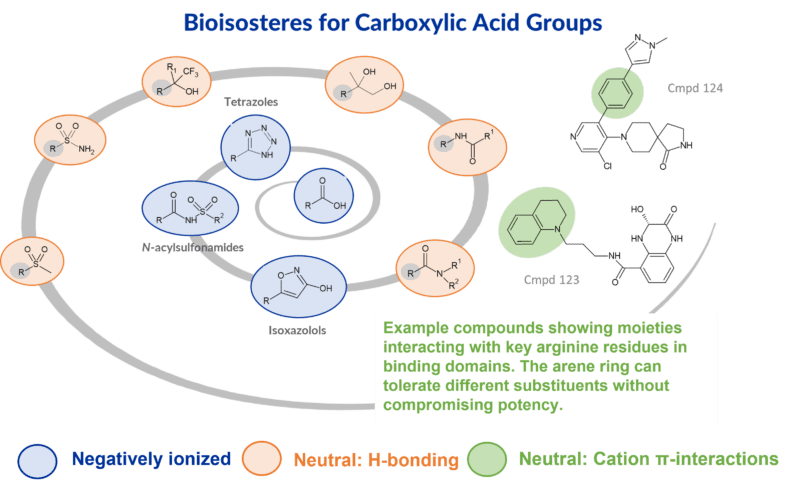
Neutral bioisosteres rely on non-ionic interactions with amino acids in the active site through hydrogen bonding and cation π-interactions. For example, an arene derived cation π-interaction with a key Arg residue in a binding site may allow replacement of a carboxylic acid pharmacophore. The aryl group does not need to be electron deficient, thus potentially alleviating any metabolic instability.
Ionized bioisosteres
- Tetrazoles – subject to N-glucuronidation or oxidative metabolism instead of acyl glucuronidation
- N-acylsulfonamides
- Isooxazolols
Neutral bioisosteres
- Hydrogen bonding interactions
- Cation π-interactions
Paper (open access)
Hall A, Chatzopoulou M, Frost J. Bioisoteres for carboxylic acids: From ionized isosteres to novel unionized replacements. Bioorg Med Chem. 2024 Mar 1;104:117653. doi: 10.1016/j.bmc.2024.117653. Epub ahead of print. PMID: 38579492.


