Metabolism-mediated loss of radiolabel during human mass balance studies
Our choice for February comprises two papers concerned with the impact of metabolism-mediated loss of radiolabel during human mass balance studies.
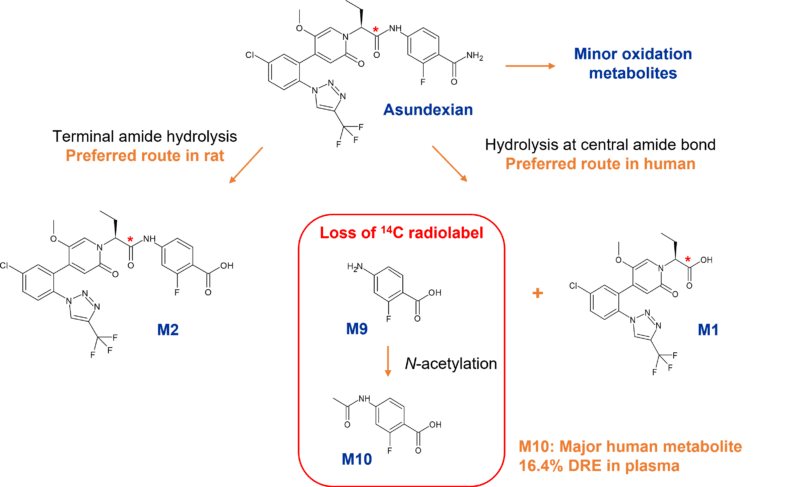
The first paper from scientists at Bayer describes the impact of metabolism-mediated hydrolysis of asundexian. Asundexian is primarily metabolised through two hydrolysis pathways with hydrolysis at the central amide bond the dominant route in human. This results in cleavage of the drug to two metabolites, M1 and M9, with only M1 retaining the 14C label. M9 is then N-acetylated to M10, a major human metabolite comprising 16.4% of total drug related material in plasma. Both M9 and M10 effectively become dark through radiodetection and instead were detected and quantified by LC-MS/MS.
The second paper looks more generally at how to approach the design of human mass balance studies where a drug compound is cleaved via degradation or metabolism resulting in loss of radiolabel in one or more metabolites.
Regulatory agencies advise that not only should the radiolabel be positioned in a chemically and metabolically stable position, in some instances, two separate positions should be used. The paper, written by a consortium of scientists from several big pharma companies, points out that discussions around multi labelling are happening with more frequency due to the increase in larger molecular weight small molecules being developed.
Biotransformation studies which reveal significant cleavage of a drug could thus trigger a route involving dual radiolabelling. This may be compounded by the increasing hydrophilicity of any cleavage products boosting the likelihood of the metabolite circulating to a higher extent and thus becoming MIST relevant. It is also noted that if LC-MS/MS is used to quantify “dark” cleavage metabolites, differences in MS responses between metabolite and the parent drug need to be considered.
The paper discourages use of multiple labels in the same molecule due to the complexity of assessing individual specific activities. Instead, if needed, separate dosing of drug labelled in different positions to separate populations is suggested as the technically easiest route. Alternative strategies such as alternative positioning of a radiolabel or following the formation of the nonlabelled metabolites using other analytical techniques are encouraged where possible.
Papers (both open access)
1. Piel I, Engelen A, Lang D et al. Metabolism and Disposition of the Novel Oral Factor XIa Inhibitor Asundexian in Rats and in Humans. Eur J Drug Metab Pharmacokinet 48, 411–425 (2023). https://doi.org/10.1007/s13318-023-00838-4
2. Cuyckens F, Hvenegaard MG, Cassidy KC et al. Recommendations on multiple label use in human ADME. Drug Metab. Dispos. January 12, 2024. https://doi.org/10.1124/dmd.123.001429


