Unusual gut metabolites
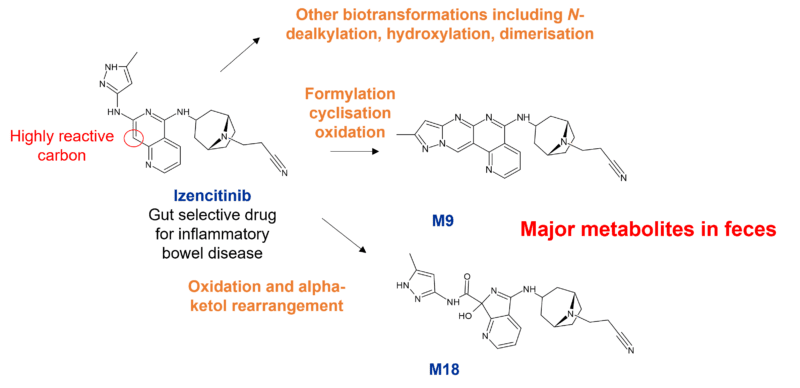
For drugs that are locally acting, prominent metabolites in the target organ may be just as significant than systemically circulating metabolites. The pan JAK inhibitor izencitinib is a gut-selective drug which is extensively metabolised, but where no one metabolite exceeded 10% of total drug-related material in plasma. However, given that izencitinib is locally acting, it was proposed in this paper that the two unusual gut metabolites (M9 and M18) which together represented more than 50% of the administered dose, were more relevant for evaluating the pharmacological and toxicological properties of the drug.
The structures of these major metabolites are unusual, resulting from oxidation and rearrangement of izencitinib to M18 with a proposed one-carbon addition with formaldehyde forming a tetracyclic product M9. Interestingly both M9 and M18 were formed in homogenised human faecal incubations, however M18 could also be formed in human liver microsomes along with M1 (N-dealkylated metabolite), M2 (homodimer of izencitinib), M3 heterodimer of izencitinib and M1), and M11 (formylated dimer).
Both metabolites were tested for pharmacological activity and were inactive. Although none of the metabolites of izencitinib described in the paper exceeded the 10% threshold in plasma, the authors point out that toxicological testing of these “local metabolites” should be the focus rather than systemic exposure, the latter being irrelevant to this locally acting drug. They recommend that more focus should be placed on metabolites in high concentrations in the local target organ for further pharmacological and toxicological evaluation, an aspect not covered by the current MIST guidance on the safety evaluation of drug metabolites.
Paper
Suresh Yeola, Ilaria Badagnani, Xiaojun Huang et al. The Metabolic Fate of Izencitinib, a Gut-selective Pan-JAK Inhibitor, in Humans. Identification of Unusual Fecal Metabolites and Implications for MIST Evaluation., 08 May 2023, PREPRINT (Version 1) available at Research Square. https://doi.org/10.21203/rs.3.rs-2815584/v1