Metabolite Tales Blog
Hypha’s “Metabolite Tales” blog articles are purely science-focussed commentary on interesting developments and papers relevant to the role of metabolism in drug discovery and development. The content is particularly relevant to those with a background in medicinal chemistry, drug metabolism and biotransformation.
We also recommend the following excellent blogs:
Drug Hunter – a trusted, independent source of curated, factual information for the drug discovery community, in particular the articles, case studies and cheat sheets!
In The Pipeline – Derek Lowe’s commentary on drug discovery and the pharma industry.
Hypha's Metabolite Tales Blog Articles
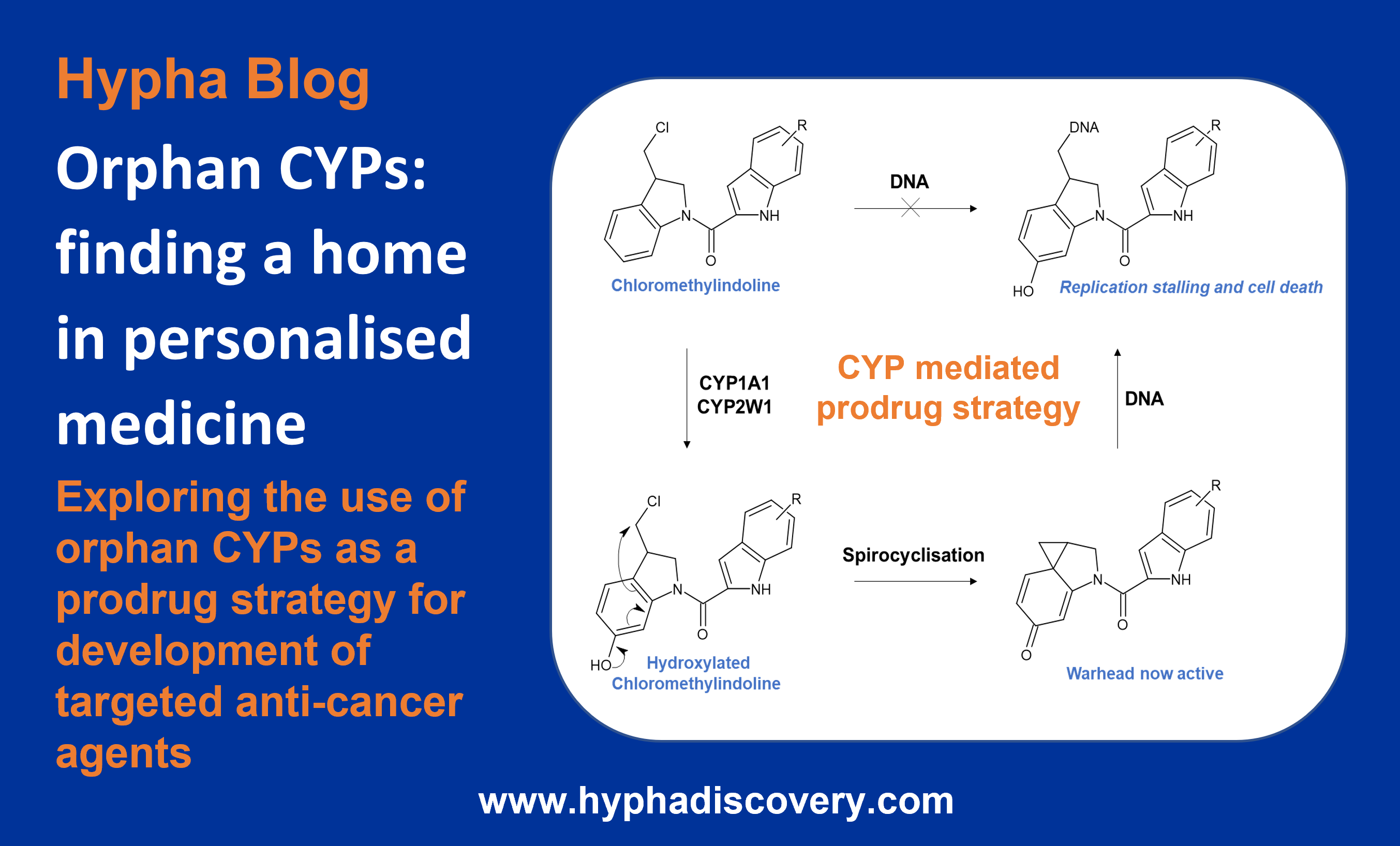
This blog highlights the prospect of exploiting orphan human CYPs for development of targeted anti-cancer agents through a prodrug strategy.
In this blog Consultant and Medicinal Chemistry expert Rob Young explores some of the potential impacts of hydroxyl groups in medicinal chemistry, contextualising opportunities alongside other fundamental structural changes and considering the physical consequences of its introduction.
Steve Djuric, Consultant and ex VP of Medicinal Chemistry at Abbvie, shares his thoughts on the challenges we face today in selecting drug candidates.
In this blog Steve delves into some of the PK demands, including permeability of PROTACs, CYP and AO metabolism, and the impact of AI.
Our guest blogger Guy Webber discusses the reasons for de-risking DMPK and why he advises integrating metabolic de-risking into drug discovery processes.
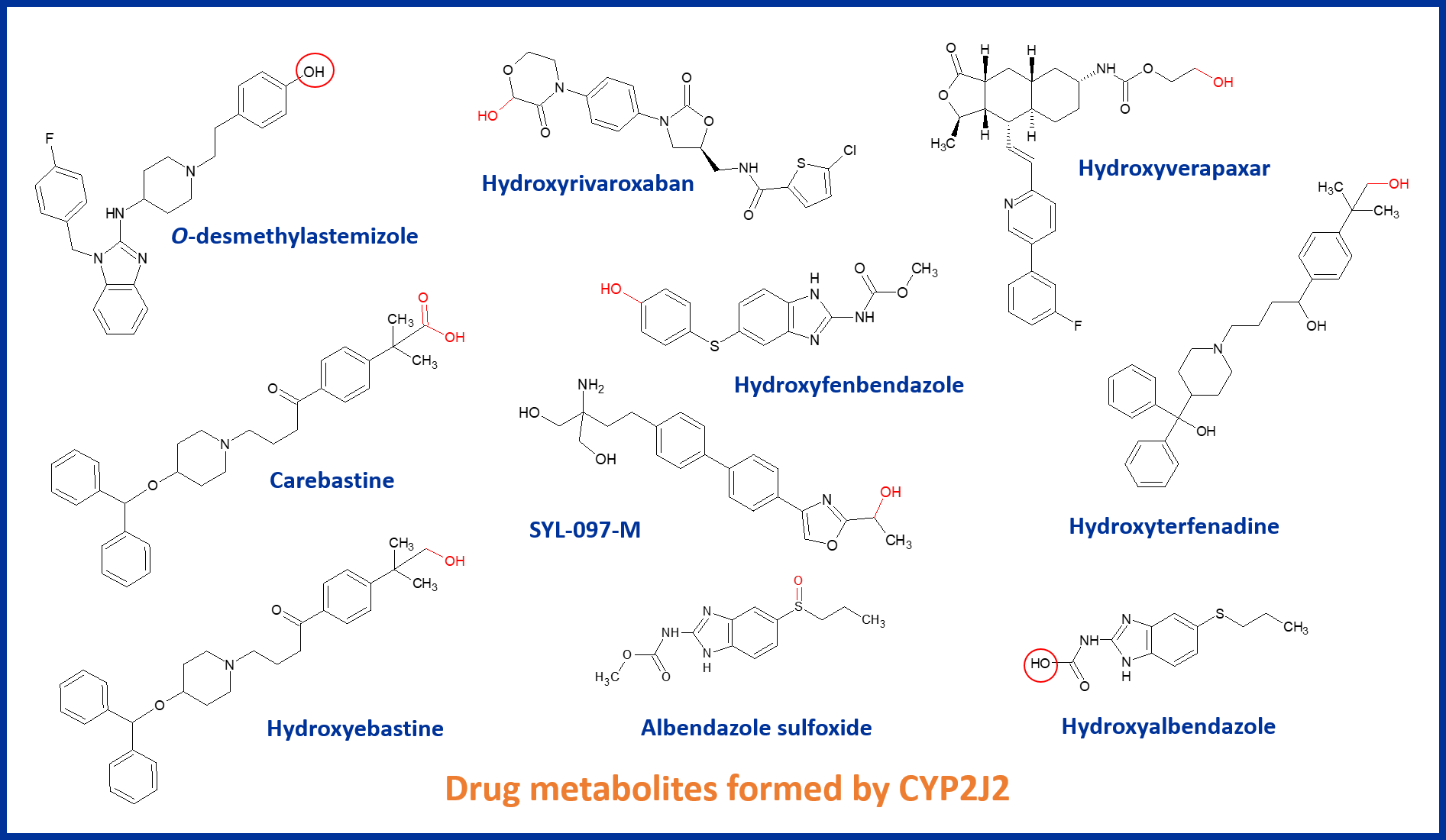
In this blog we explore the relevance of the mainly extrahepatic epoxygenase CYP2J2. Given it’s role at the crossroads of drug metabolism and endogenous bioactive oxy-lipid synthesis, we discuss whether there is a case for greater importance to be placed on this CYP.
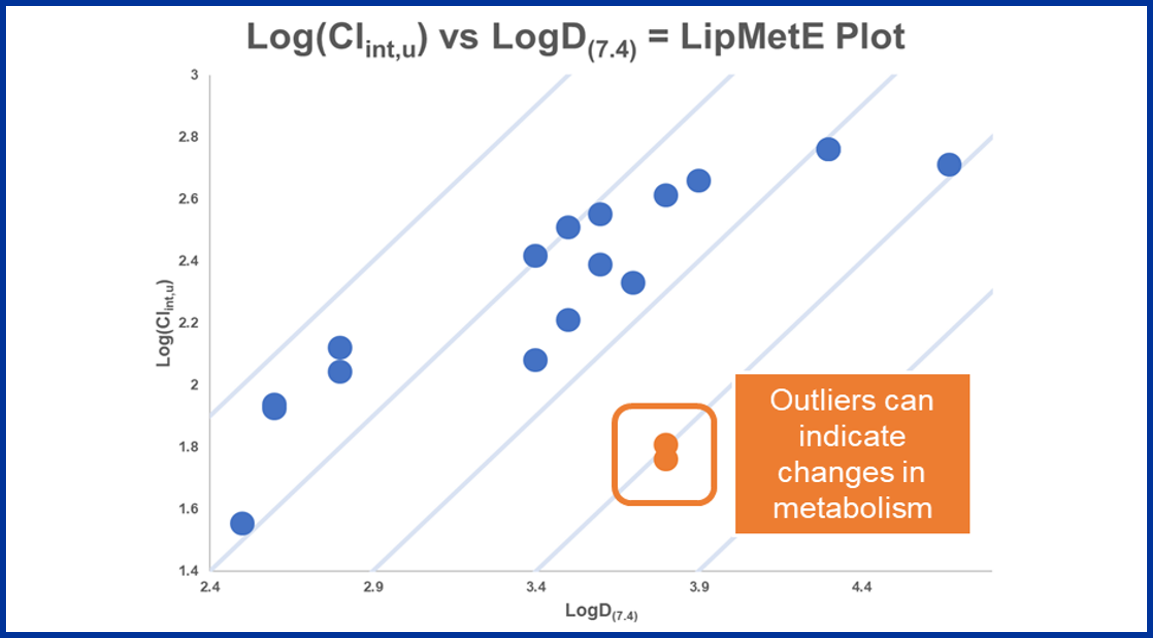
LipMetE is a potentially useful and underutilised efficiency metric that relates logD to microsomal clearance. In this blog post we explore its origins and application to analyse metabolism trends in drug design.
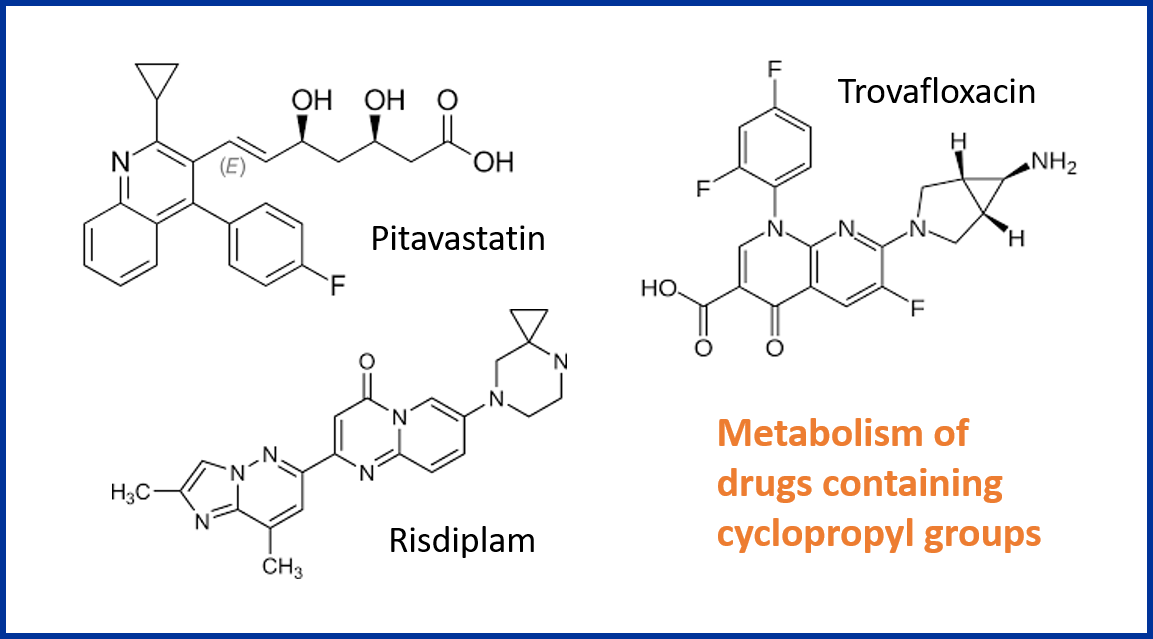
Cyclopropyl groups are an appealing substituent to use in drug design because they impart constraint in aliphatic systems while retaining a high fraction of sp3. Furthermore, the ring strain produces shorter, stronger, more polarised C-H bonds that impart some novel properties, not least the reduced susceptibility to oxidative metabolism by cytochrome P450 (CYP) enzymes. In this blog post we explore the metabolism of various drug structures that contain cyclopropyl groups, revealing that cyclopropyl groups can be used advantageously to diminish oxidative metabolism in some circumstances, but when coupled to amines can lead to potentially reactive intermediates.
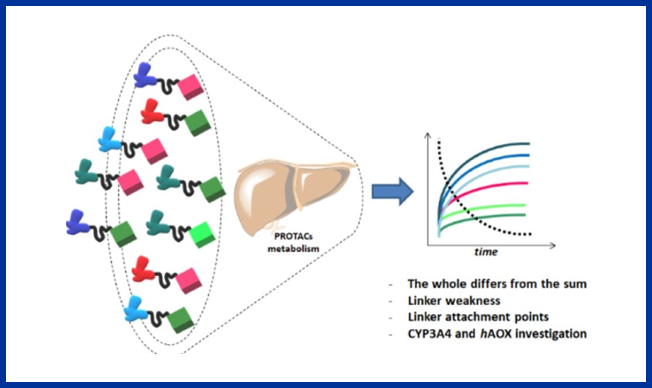
An understanding of the metabolism of PROTACs is a growing need since their metabolism can’t be readily predicted from the individual ligands that constitute the chimera. A paper by Goracci et al. looks at the metabolic stability of a collection of PROTACs in cryopreserved human hepatocytes, and vs CYP3A4 and aldehyde oxidase. The linker appears to be the most labile moiety, with any instability localized at the attachment points. Depending on the type of linker, O- and N-dealkylations were observed.
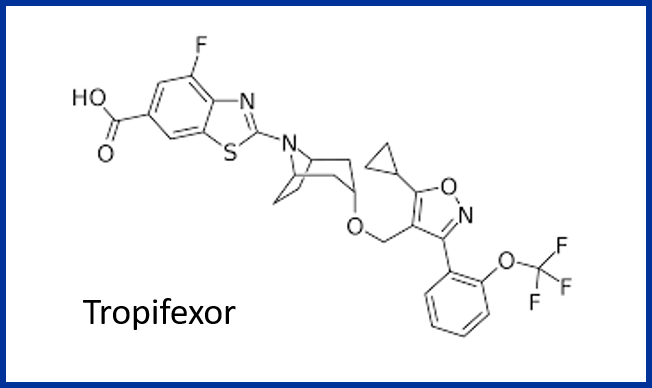
Looking at the structure of tropifexor, a farnesoid X receptor (FXR) agonist for the treatment of nonalcoholic steatohepatitis (NASH), immediately raises thoughts about formation of an acyl glucuronide metabolite. In fact, tropifexor is predominantly eliminated by metabolism and indeed the acyl glucuronide (M22) was observed during in vitro studies, although not in the human radiolabelled ADME study described in the paper by scientists at Novartis (see reference below).
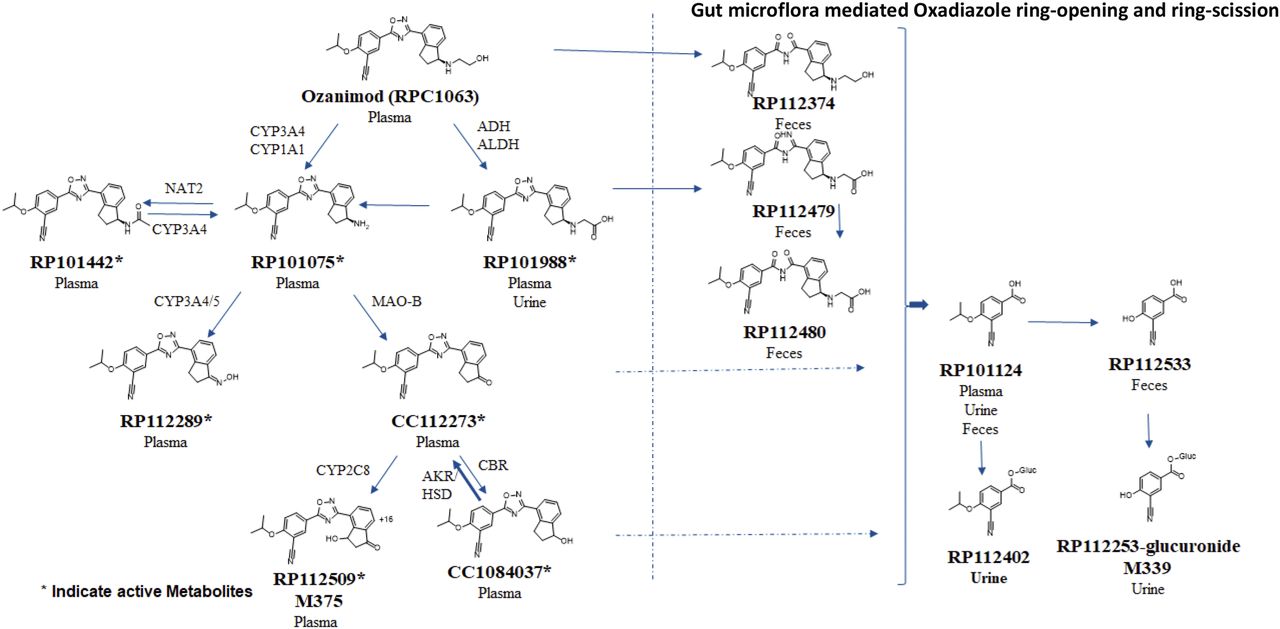
Check out the implications of the complex metabolism of ozanimod discussed in a paper from Sekhar Surapaneni and colleagues at BMS. There are a plethora of elements involved in metabolism of the drug including: The uncovering of active major circulating and disproportionate human metabolites late in development. The involvement of multiple non-CYP phase 1 mechanisms. The contribution of gut microbes.
Here’s a great paper published by Jasleen Sodhi and Leslie Benet discussing the difficulties surrounding the prediction of in vivo clearance, in particular the IVIVE (in vitro to in vivo extrapolation) underprediction which can vary significantly from drug-to-drug. Hepatic clearance largely determines the exposure of drug in the body, influencing both the efficacy and safety of a new drug, and thus it’s important to get the prediction correct! IVIVE is the most commonly employed methodology to predict hepatic clearance.
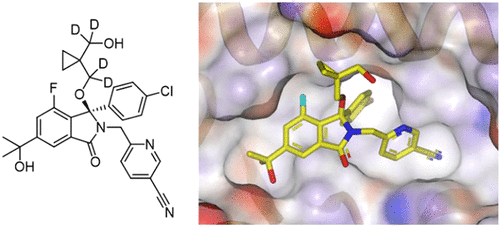
Multi-faceted paper from the team at Astex Pharmaceuticals (UK) and Cancer Research UK Newcastle Drug Discovery Unit describing the rational design of novel isoindolinone inhibitors of the p53-MDM2 protein-protein interaction. What’s particularly interesting was the application of deuterium to alleviate oxidation at a metabolic hot spot as well as reducing intestinal CYP3A4 metabolism to maximize oral bioavailability.
Stay up to date with the latest news from Hypha Discovery
Sign up for our quarterly newsletters and monthly "Metabolite Tales" blog
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk