Metabolite Tales Blog
Hypha’s “Metabolite Tales” blog articles are purely science-focussed commentary on interesting developments and papers relevant to the role of metabolism in drug discovery and development. The content is particularly relevant to those with a background in medicinal chemistry, drug metabolism and biotransformation.
We also recommend the following excellent blogs:
Drug Hunter – a trusted, independent source of curated, factual information for the drug discovery community, in particular the articles, case studies and cheat sheets!
In The Pipeline – Derek Lowe’s commentary on drug discovery and the pharma industry.
Hypha's Metabolite Tales Blog Articles
In this next instalment we focus on drugs where metabolism in humans results in circulating glucuronides and other conjugates.
Over the next couple of months we’ll be taking a look at the metabolism of small molecule drugs approved by the FDA in 2023, starting with those where there is mention of the involvement of active metabolites.
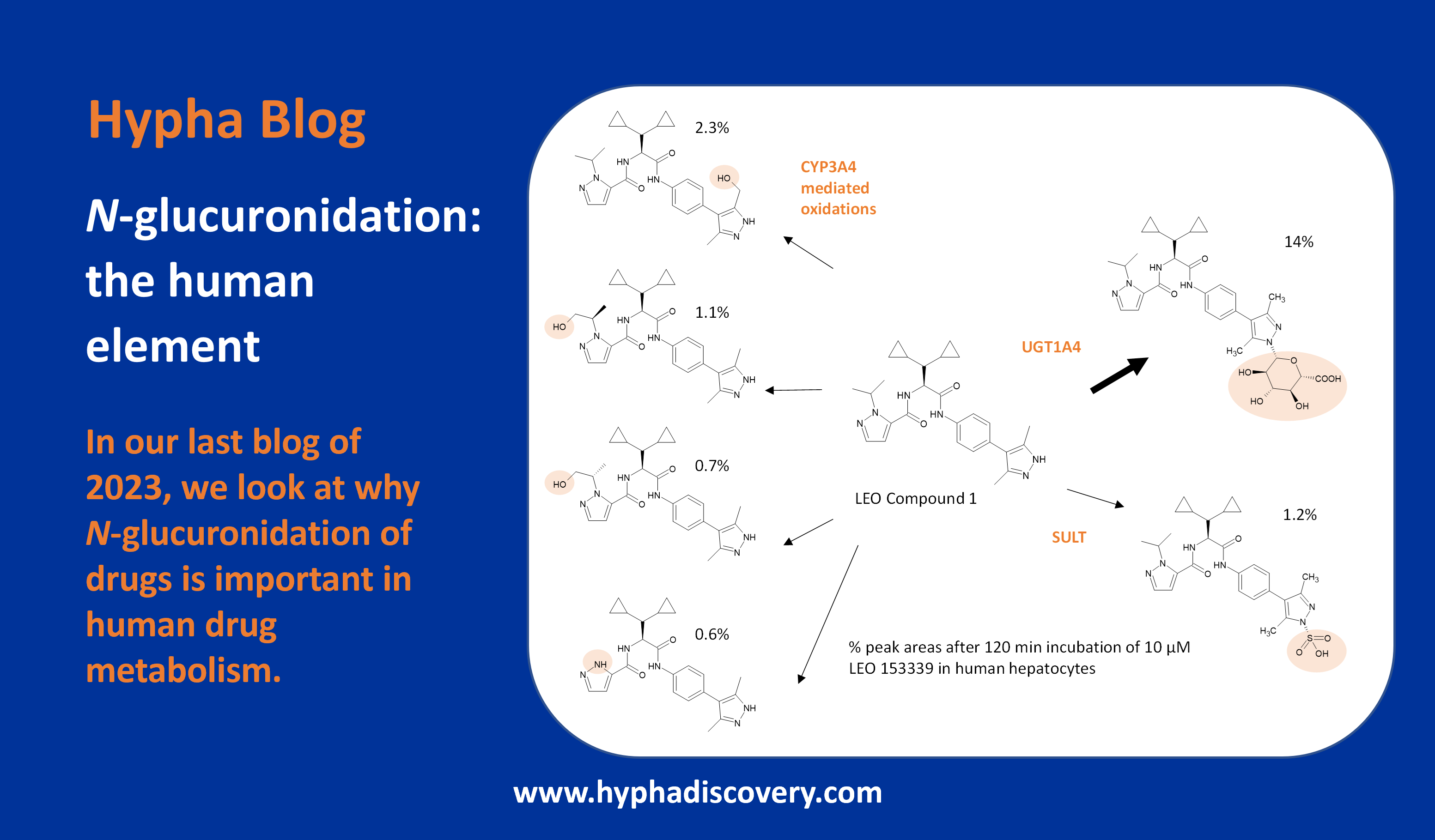
In our last blog of the year, we look at why N-glucuronidation of small molecule drugs is important in human drug metabolism.
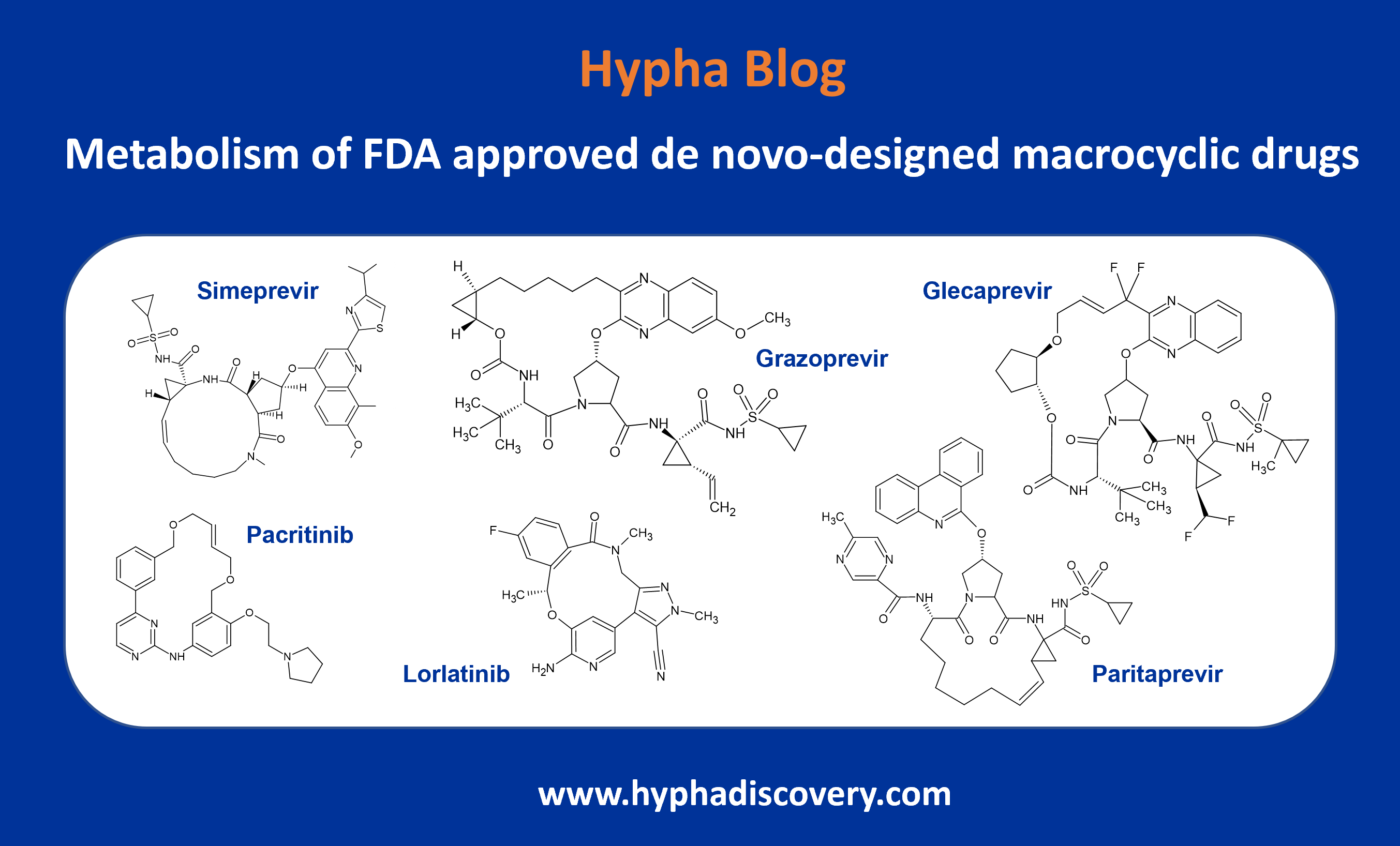
In this blog we delve into the metabolism of selected de novo-designed macrocyclic drugs approved by the FDA.
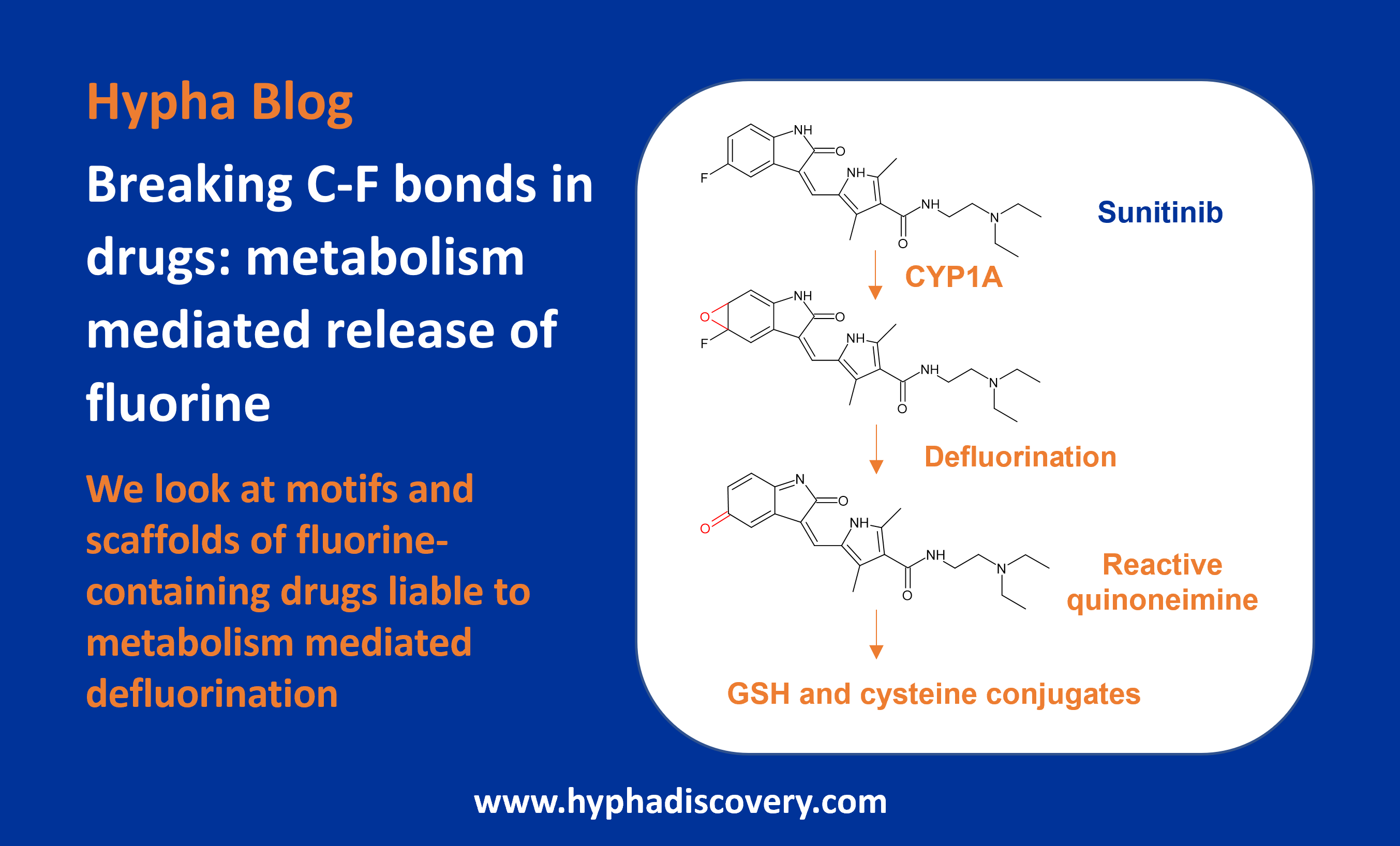
In this blog we look at motifs and scaffolds of F-containing drugs that are subject to metabolism mediated defluorination, along with some drug design strategies that have been used to circumvent this.
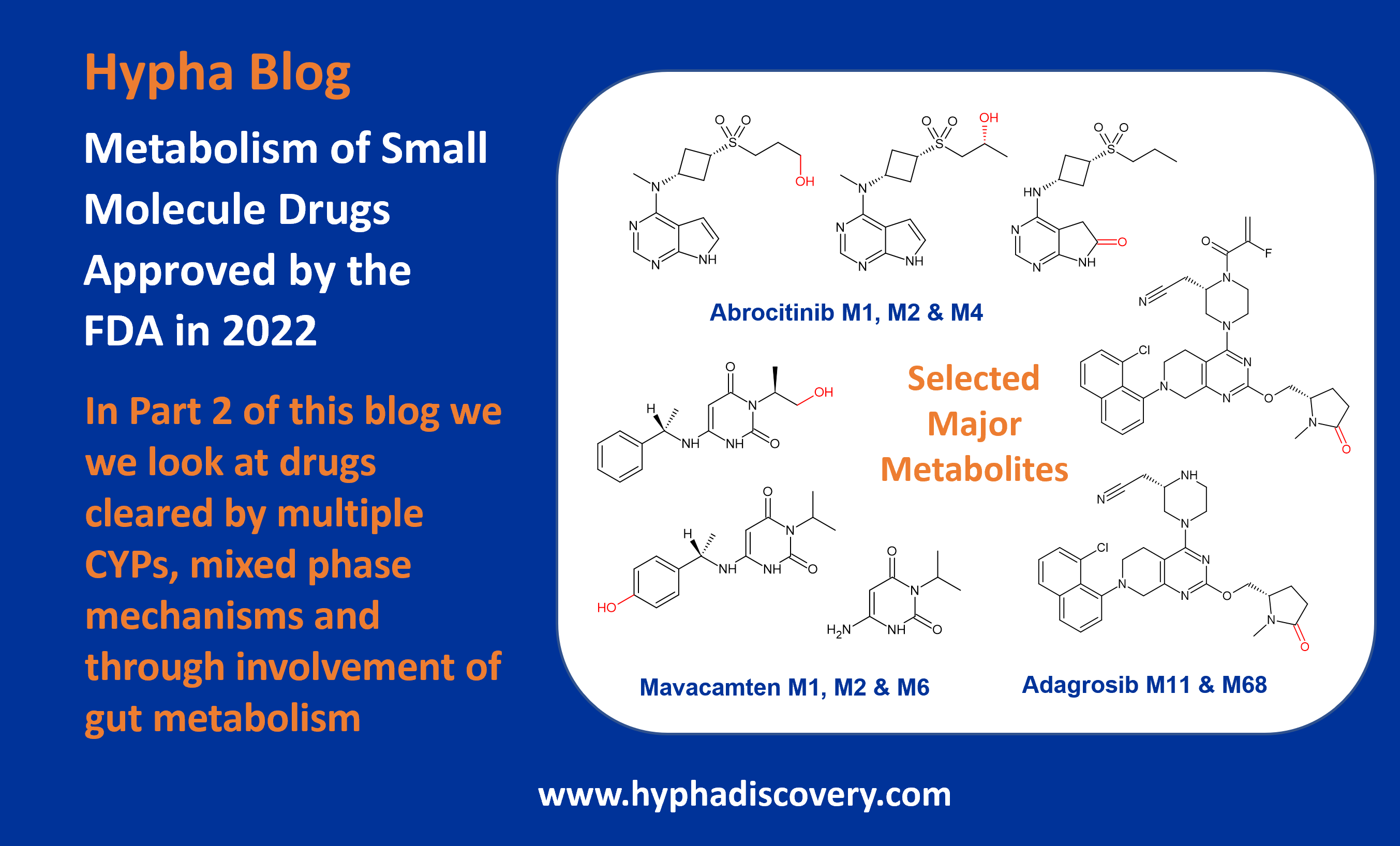
In this follow-up blog, we dig into the drugs approved by the FDA in 2022 that are cleared by multiple CYPs, mixed phase mechanisms and through involvement of gut metabolism.
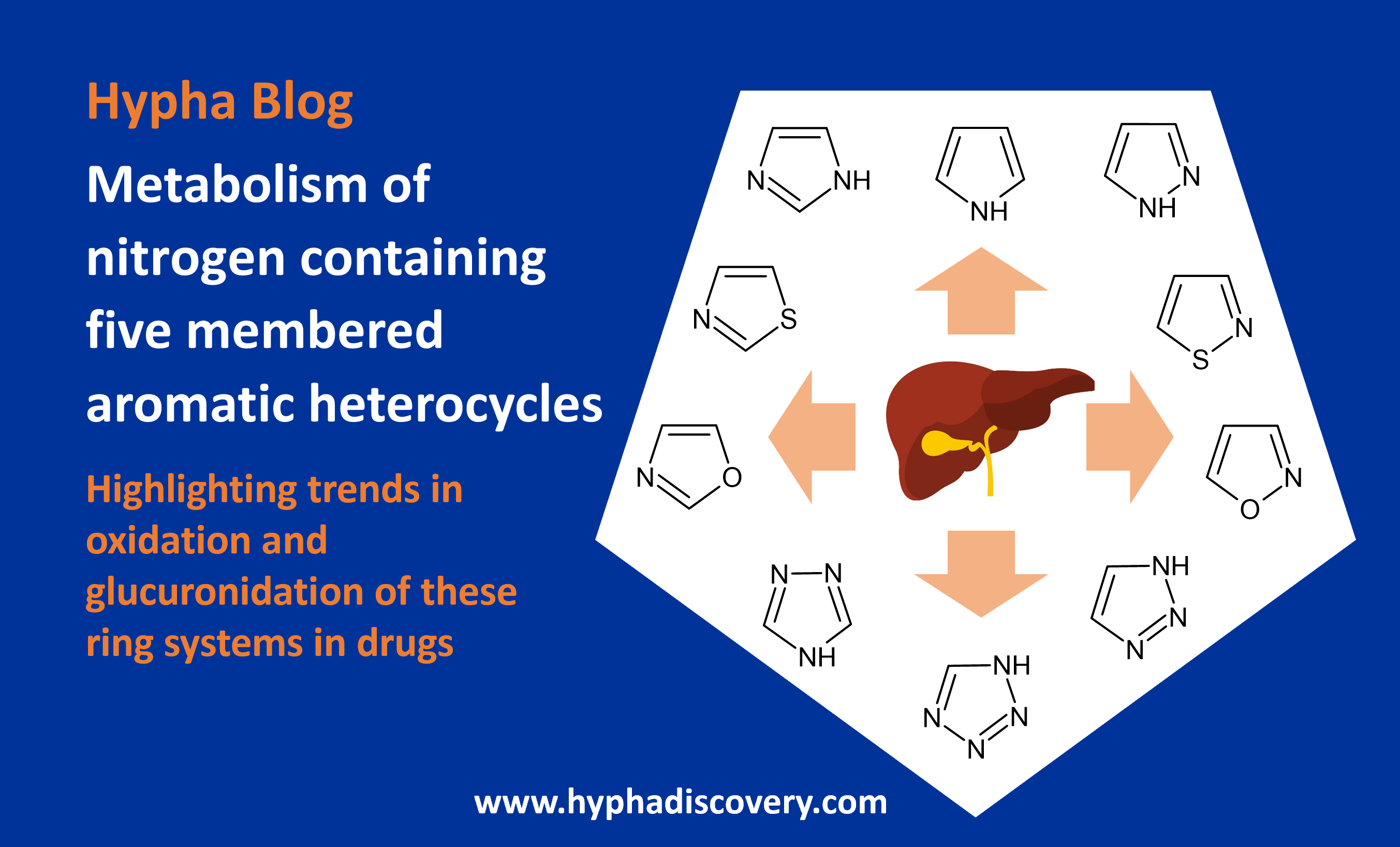
Our blog this month highlights trends in metabolism of five membered nitrogen containing aromatic heterocycles in drug compounds.
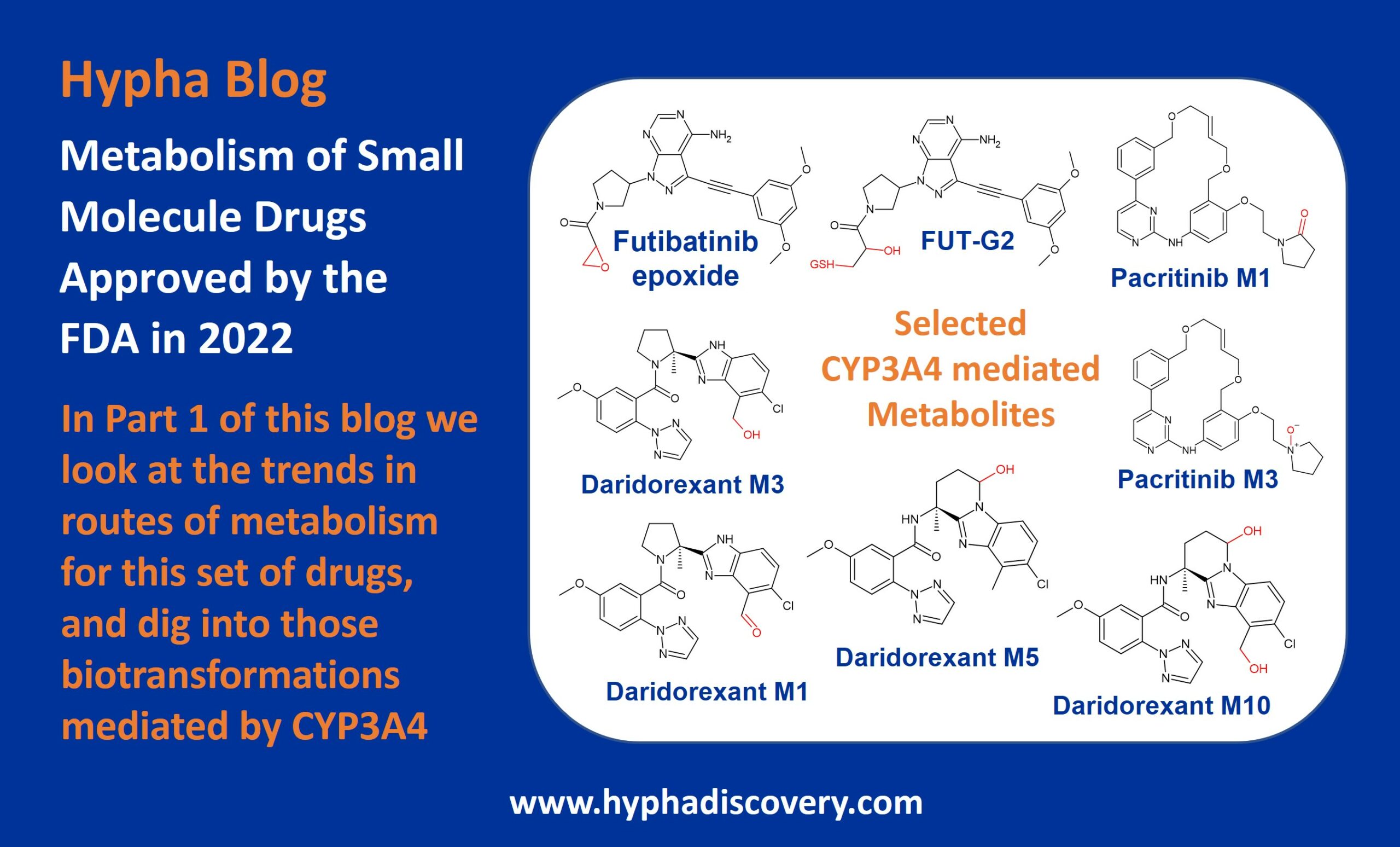
In Part 1 of this blog on metabolism of small molecules approved by the FDA in 2022 we look at the trends, and whether CYP3A4 still rules.
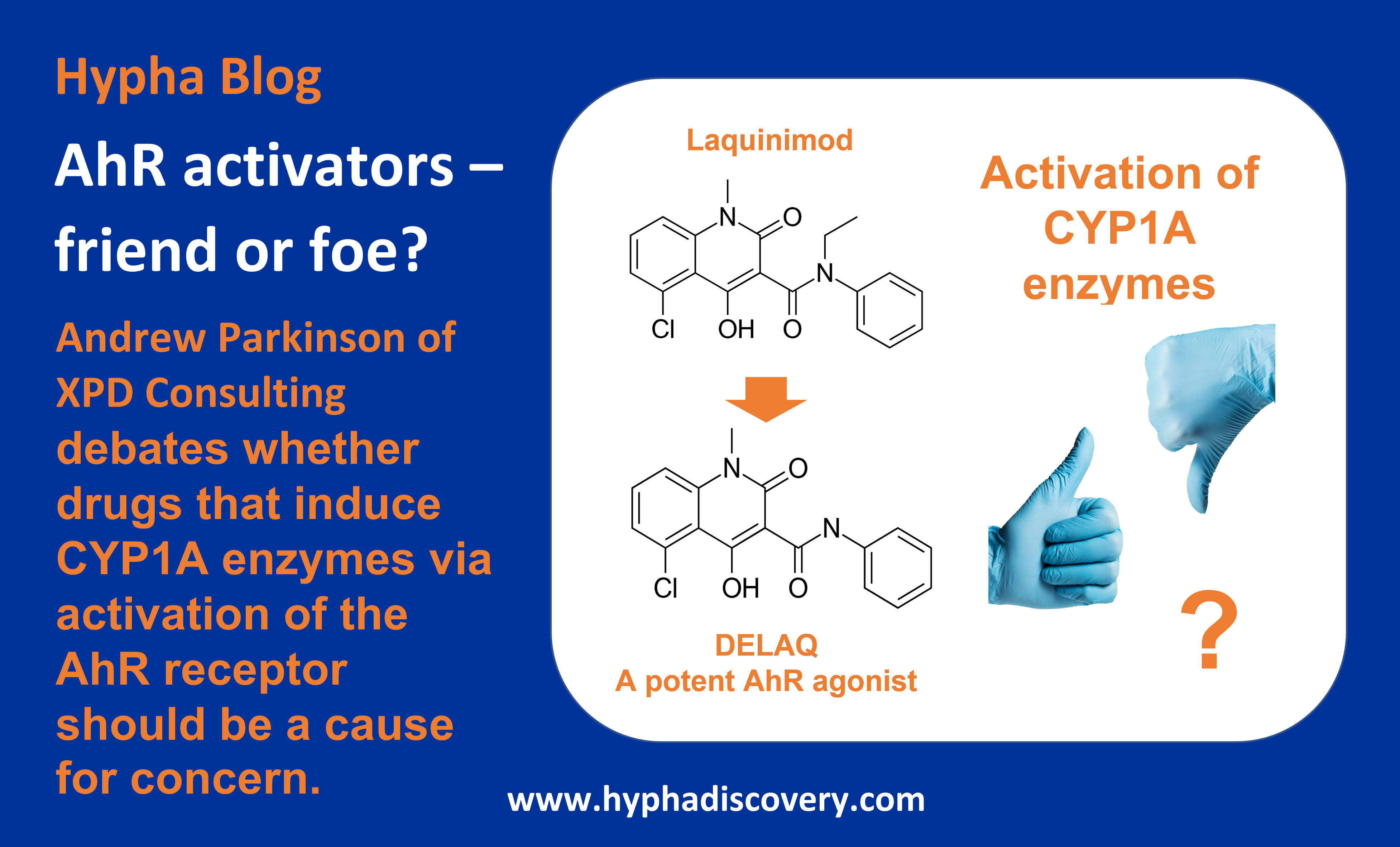
Andrew Parkinson, Consultant and former Founder and CSO/CEO at Xenotech, debates whether drugs that induce CYP1A enzymes via activation of the AhR receptor should be a cause for concern or not.
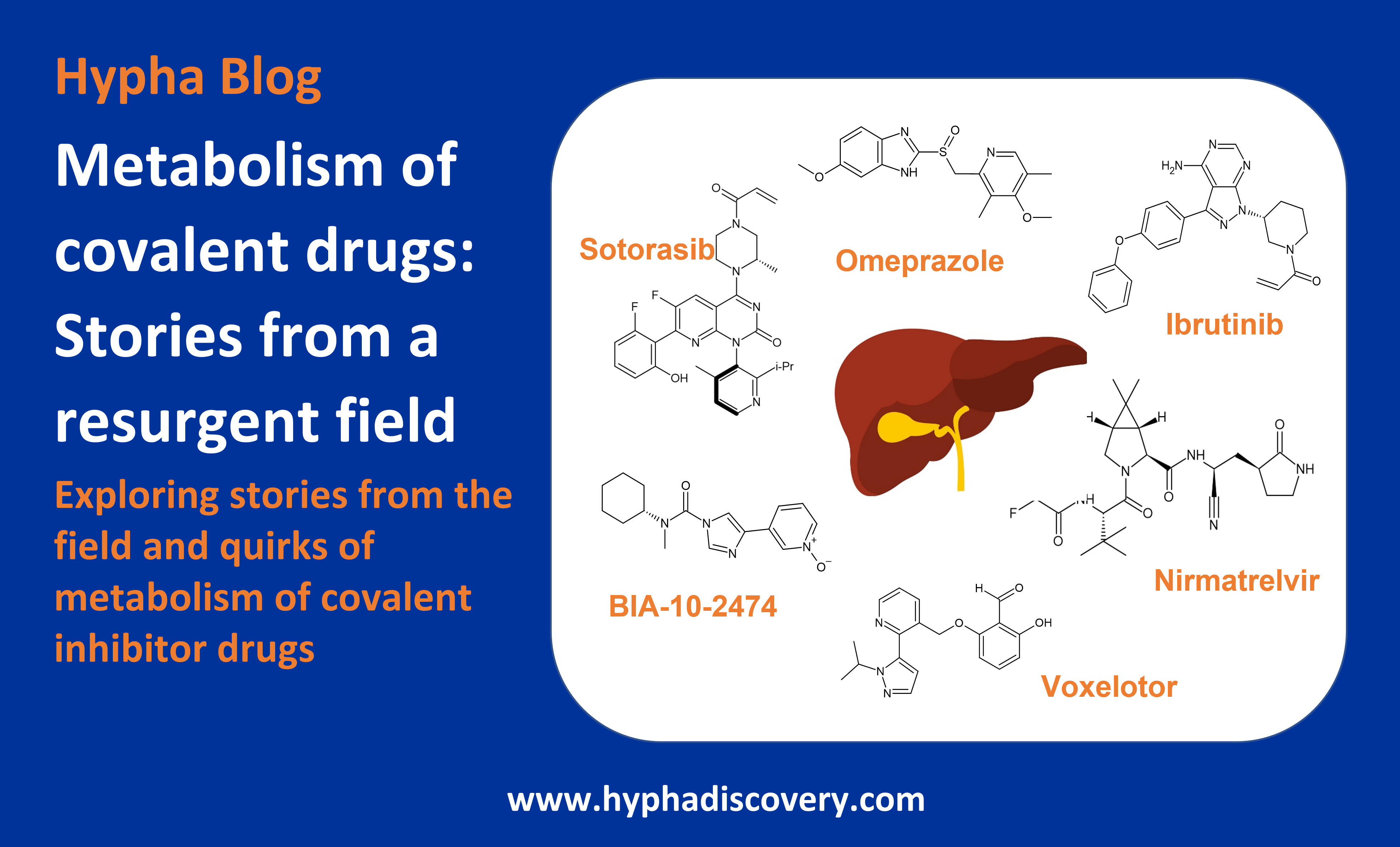
In this blog we look at metabolism of covalent drugs, highlighting some interesting stories from the field and metabolism quirks of covalent inhibitors.
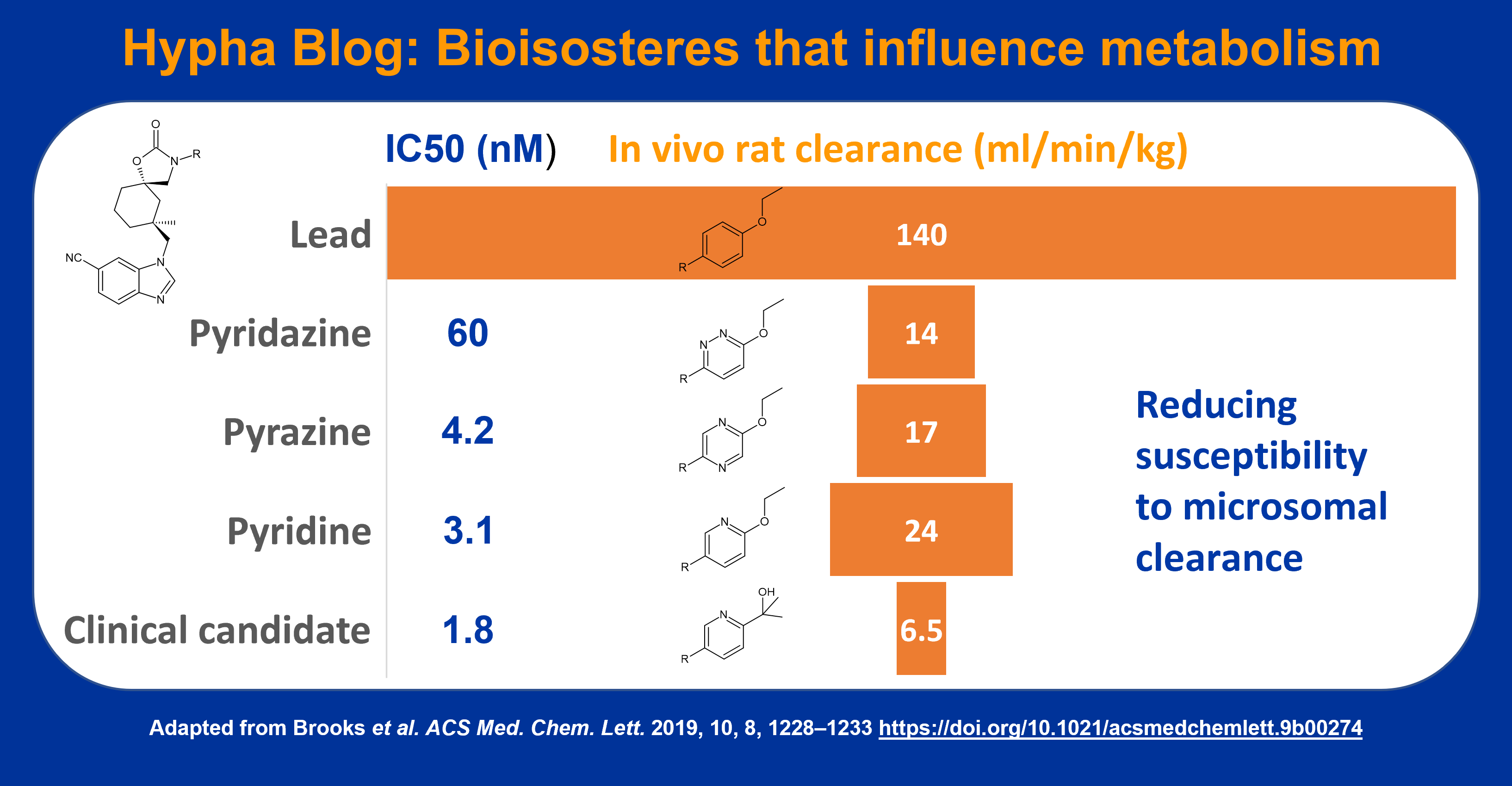
The majority of reviews on the application of bioisosteres are focused on effects on potency. In this blog we focus on how bioisosteres can alter metabolism of a drug.
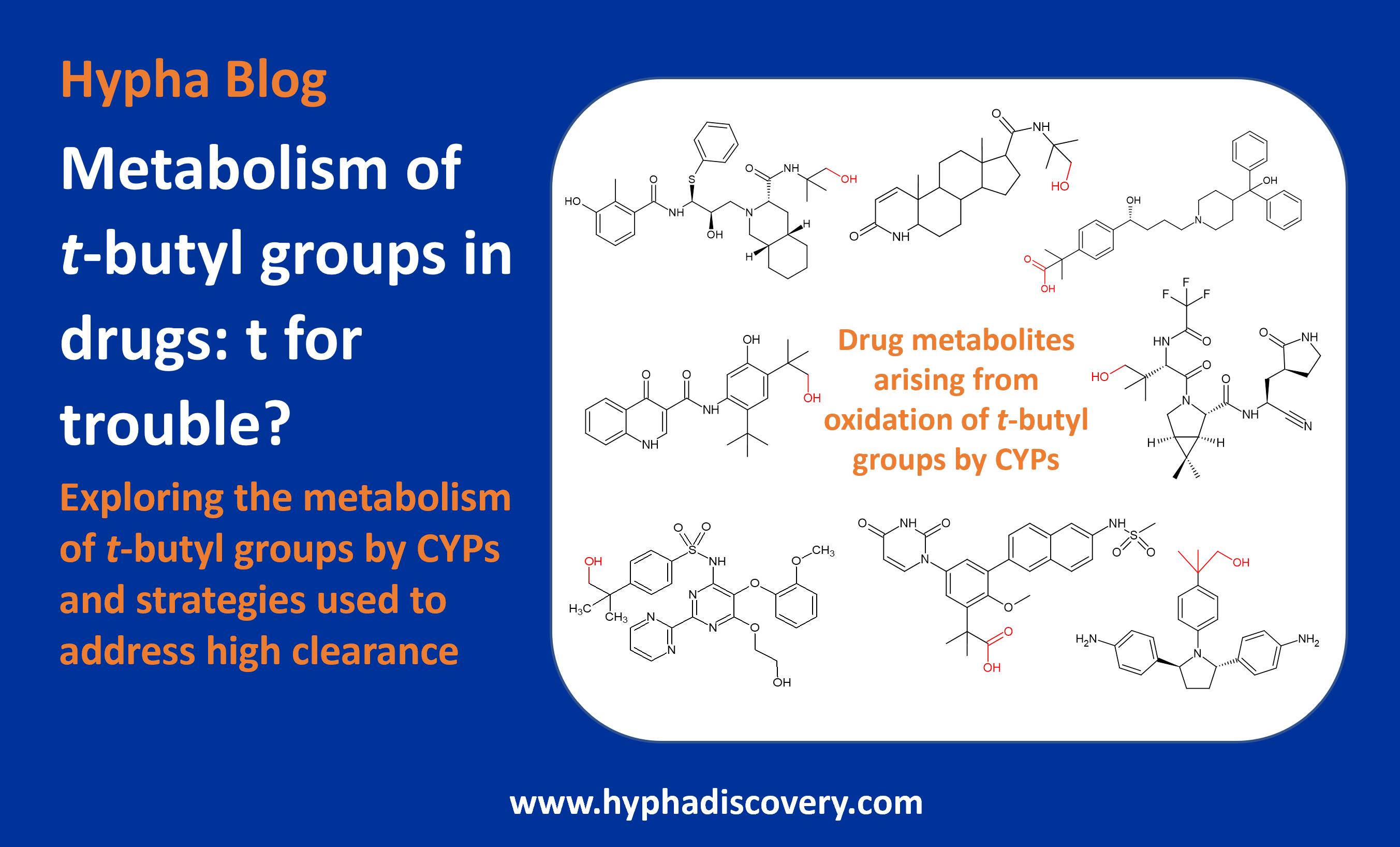
Here we discuss metabolism of drugs in which CYPs catalyse oxidation of tert-butyl groups.
Stay up to date with the latest news from Hypha Discovery
Sign up for our quarterly newsletters and monthly "Metabolite Tales" blog
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk